109451
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

CYP2D6 phenotypes are associated with adverse outcomes related to opioid medications
Authors St Sauver JL, Olson JE, Roger VL, Nicholson WT, Black III JL, Takahashi PY, Caraballo PJ, Bell EJ, Jacobson DJ, Larson NB, Bielinski SJ
Received 7 March 2017
Accepted for publication 6 June 2017
Published 24 July 2017 Volume 2017:10 Pages 217—227
DOI https://doi.org/10.2147/PGPM.S136341
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Akshita Wason
Peer reviewer comments 2
Editor who approved publication: Dr Martin Bluth
Background: Variation in the CYP2D6 gene
may affect response to opioids in both poor and ultrarapid metabolizers, but
data demonstrating such associations have been mixed, and the impact of
variants on toxicity-related symptoms (e.g., nausea) is unclear. Therefore, we
examined the association between CYP2D6 phenotype
and poor pain control or other adverse symptoms related to the use of opioids
in a sample of primary care patients.
Materials and
methods: We identified all patients in
the Mayo Clinic RIGHT Protocol who were prescribed an opioid medication between
July 01, 2013 and June 30, 2015, and categorized patients into three
phenotypes: poor, intermediate to extensive, or ultrarapid CYP2D6 metabolizers. We
reviewed the electronic health record of these patients for indications of poor
pain control or adverse symptoms related to medication use. Associations
between phenotype and outcomes were assessed using Chi-square tests and
logistic regression.
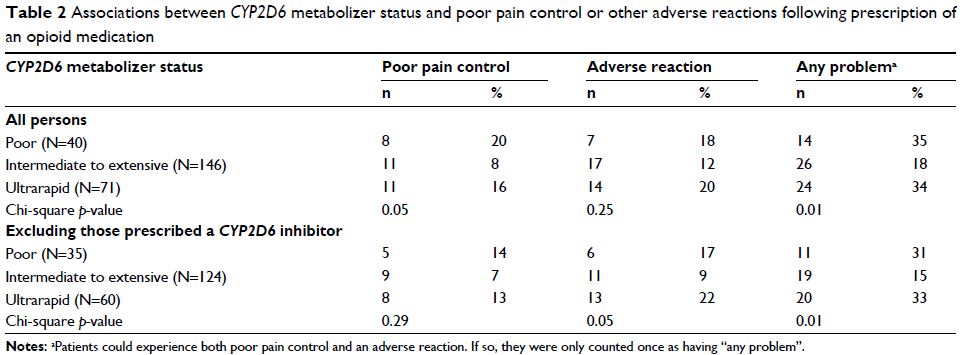
Results: Overall, 257 (25% of RIGHT Protocol participants) patients received at
least one opioid prescription; of these, 40 (15%) were poor metabolizers, 146
(57%) were intermediate to extensive metabolizers, and 71 (28%) were ultrarapid
metabolizers. We removed patients that were prescribed a CYP2D6 inhibitor medication
(n=38). After adjusting for age and sex, patients with a poor or ultrarapid
phenotype were 2.7 times more likely to experience either poor pain control or
an adverse symptom related to the prescription compared to patients with an
intermediate to extensive phenotype (odds ratio: 2.68; 95% CI: 1.39,
5.17; p =0.003).
Conclusion: Our results suggest that >30% of patients with a poor or
ultrarapid CYP2D6 phenotype may experience
an adverse outcome after being prescribed codeine, tramadol, oxycodone, or
hydrocodone. These medications are frequently prescribed for pain relief, and
~39% of the US population is expected to carry one of these phenotypes,
suggesting that the population-level impact of these gene–drug interactions
could be substantial.
Keywords: CYP2D6, opioid, phenotype, adverse effects