109451
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Etanercept (Enbrel®) alternative storage at ambient temperature
Authors Shannon E, Daffy J, Jones H, Paulson A, Vicik SM
Received 7 January 2017
Accepted for publication 8 June 2017
Published 21 July 2017 Volume 2017:9 Pages 87—99
DOI https://doi.org/10.2147/CPAA.S131832
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 3
Editor who approved publication: Professor Arthur Frankel
Background: Biologic disease-modifying antirheumatic drugs, including tumor
necrosis factor inhibitors such as etanercept (Enbrel®), have improved outcomes for patients with
rheumatic and other inflammatory diseases, with sustained remission being the
optimal goal for patients with rheumatoid arthritis. Flexible and convenient
treatment options, compatible with modern lifestyle, are important in helping
patients maintain treatment and manage their disease. Etanercept drug product
(DP) is available in lyophilized powder (Lyo) for solution injection, prefilled
syringe, and prefilled pen presentations and is typically stored under
refrigerated conditions. We aimed to generate a comprehensive analytical data
package from stability testing of key quality attributes, consistent with
regulatory requirements, to determine whether the product profile of etanercept
is maintained at ambient temperature.
Methods: Test methods assessing key attributes of purity,
quality, potency, and safety were performed over time, following storage of
etanercept DP presentations under a range of conditions.
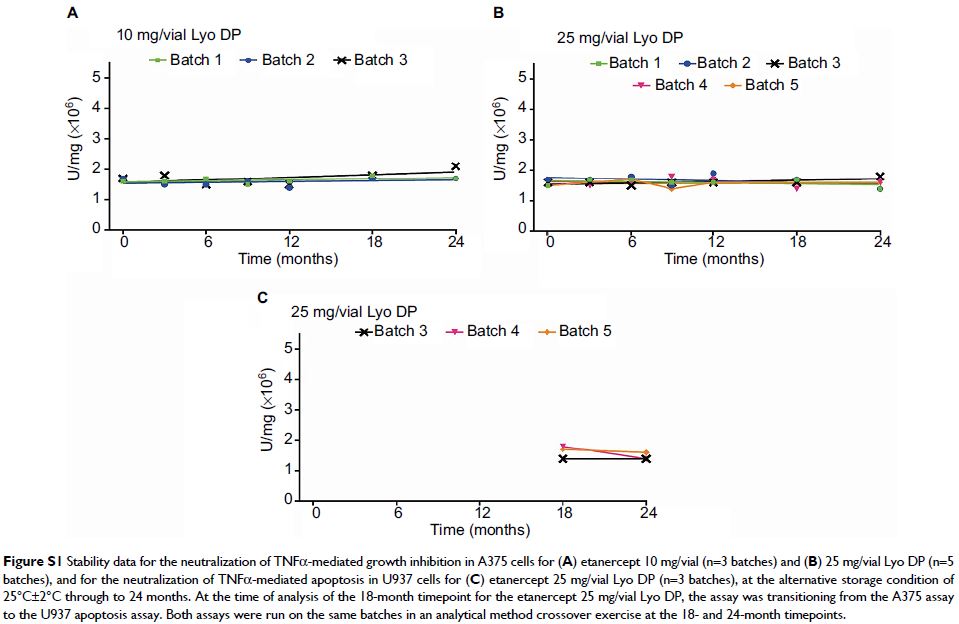
Results: Results and statistical analysis from stability
testing (based on size exclusion high-performance liquid chromatography,
hydrophobic interaction chromatography, and sodium dodecyl sulfate-polyacrylamide
gel electrophoresis Coomassie) across all etanercept presentations (10 and 25
mg/vial Lyo DP; 25 and 50 mg prefilled syringe DP; 50 mg prefilled pen DP)
showed key stability-indicating parameters were within acceptable limits
through the alternative storage condition of 25°C±2°C for 1 month.
Conclusion: Stability testing performed in line with
regulatory requirements supports a single period of storage for etanercept DP
at an alternative storage condition of 25°C±2°C for up to 1 month within the
approved expiry of the product. This alternative storage condition represents
further innovation in the etanercept product lifecycle, providing greater
flexibility and enhanced overall convenience for patients.
Keywords: etanercept,
ambient, storage, temperature, stability