109451
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

临床靶向的重组免疫毒素用于癌症治疗
Authors Li M, Liu ZS, Liu XL, Hui Q, Lu SY, Qu LL, Li YS, Zhou Y, Ren HL, Hu P
Received 13 February 2017
Accepted for publication 10 May 2017
Published 20 July 2017 Volume 2017:10 Pages 3645—3665
DOI https://doi.org/10.2147/OTT.S134584
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Akshita Wason
Peer reviewer comments 2
Editor who approved publication: Dr Carlos Vigil Gonzales
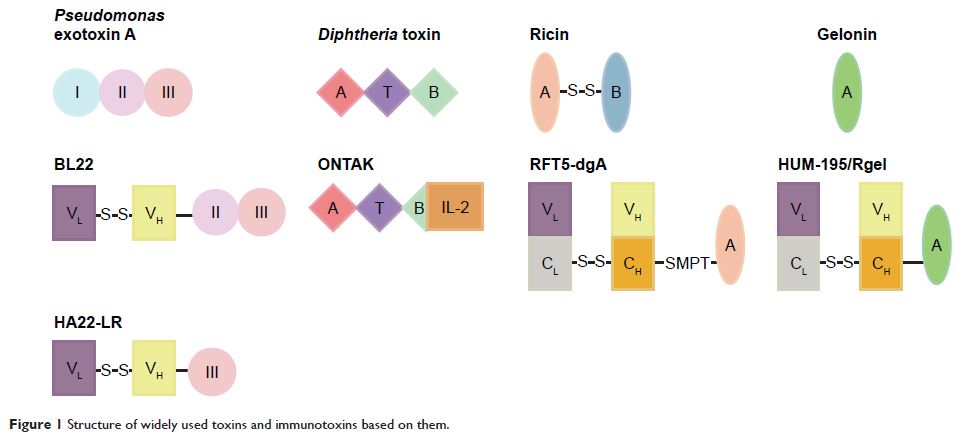
Abstract: Recombinant immunotoxins (RITs) are proteins that contain a toxin
fused to an antibody or small molecules and are constructed by the genetic
engineering technique. RITs can bind to and be internalized by cells and kill
cancerous or non-cancerous cells by inhibiting protein synthesis. A wide
variety of RITs have been tested against different cancers in cell culture,
xenograft models, and human patients during the past several decades. RITs have
shown activity in therapy of several kinds of cancers, but different levels of
side effects, mainly related to vascular leak syndrome, were also observed in
the treated patients. High immunogenicity of RITs limited their long-term or
repeat applications in clinical cases. Recent advances in the design of
immunotoxins, such as humanization of antibody fragment, PEGylation, and modification
of human B- and T-cell epitopes, are overcoming the above mentioned problems,
which predict the use of these immunotoxins as a potential therapeutic method
to treat cancer patients.
Keywords: targeted
therapy, hematologic malignancies, solid tumors, vascular leak syndrome,
immunogenicity