109451
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

使用地佐辛 (Dezocine) 预防依托咪酯 (etomidate) 诱发的肌阵挛:对随机试验的一个综合分析
Authors Zhu Y, Yang Y, Zhou C, Bao Z
Received 19 March 2017
Accepted for publication 15 May 2017
Published 18 July 2017 Volume 2017:11 Pages 2163—2170
DOI https://doi.org/10.2147/DDDT.S137464
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Salvatore Bongarzone
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Objective: This study was designed to
evaluate the efficacy and safety of preinjection of dezocine in preventing
etomidate-induced myoclonus.
Methods: PubMed,
Embase, The Cochrane Library, and China National Knowledge Infrastructure
(CNKI) were searched to collect relevant randomized controlled trials (RCTs)
from inception to July 2016 on the preinjection of dezocine in preventing
etomidate-induced myoclonus. Two researchers independently screened literature,
extracted data, and evaluated bias risks in accordance with inclusion and
exclusion criteria, and then used RevMan 5.2 to perform the
meta-analysis.
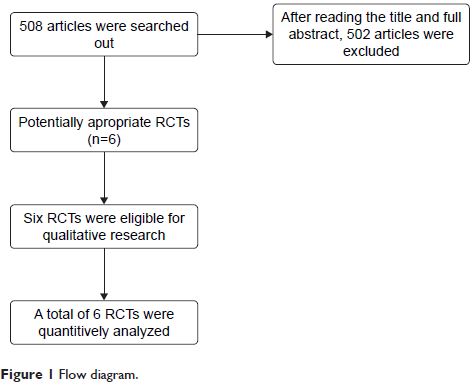
Results: A total
of six RCTs were included in this study. The meta-analysis showed that 1) the
preinjection of dezocine can reduce the incidence of etomidate-induced
myoclonus (relative risk [RR] =0.25, 95% CI [0.13, 0.50], P <0.0001), which is consistent
with the result of subgroup analysis; 2) the preinjection of dezocine can
reduce the incidence of mild, moderate, and severe myoclonus; 3) dezocine was
not related to an increasing incidence of etomidate-induced dizziness and nausea
(RR =2.83, 95% CI [0.66, 12.08], P =0.6); and 4)
dezocine did not reduce heart rates after the administration of etomidate (mean
difference =1.06, 95% CI [–4.08, 6.19], P =0.69).
Conclusion: The
preinjection of dezocine has the effect of both lowering the incidence of
etomidate-induced myoclonus and easing the severity of myoclonus, but without
increasing dizziness and nausea or affecting the heart rate.
Keywords: dezocine,
etomidate, myoclonus, meta-analysis, RCTs