109451
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Phenytoin and carbamazepine in trigeminal neuralgia: marketing-based versus evidence-based treatment
Authors Keppel Hesselink JM, Schatman ME
Received 16 May 2017
Accepted for publication 17 May 2017
Published 17 July 2017 Volume 2017:10 Pages 1663—1666
DOI https://doi.org/10.2147/JPR.S141896
Checked for plagiarism Yes
Introduction
Most review articles support carbamazepine as a
first-line pharmacotherapy for idiopathic trigeminal neuralgia.1–3 However, the empirical support for this
recommendation is somewhat suspect. Phenytoin, as the prototype for all
anticonvulsants, was already positioned as an analgesic compound 70 years ago.
Since these initial findings, the data that have been gathered have supported the
use of anticonvulsants as painkillers – from phenytoin up to and including more
recent anticonvulsants such as gabapentin and pregabalin. Since 1942, a number
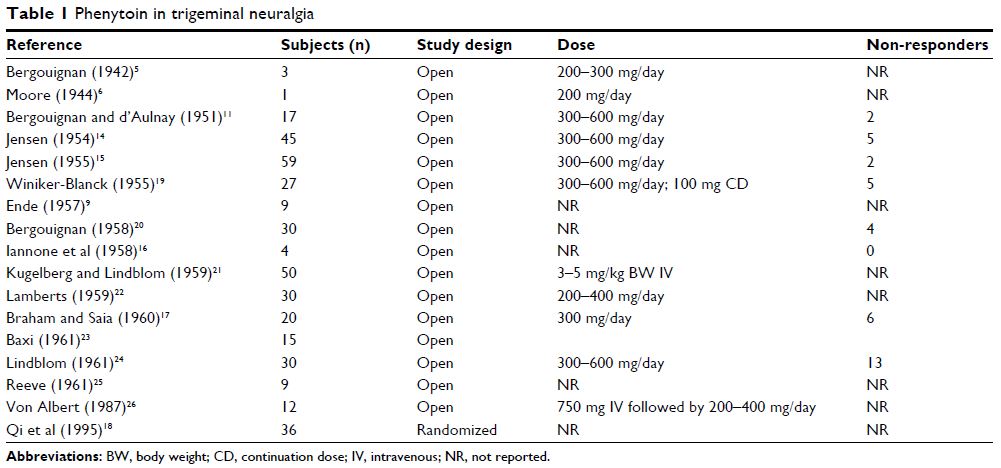
of papers supported phenytoin’s therapeutic effects in trigeminal neuralgia
(Table 1). The introduction of carbamazepine in 1962 by Geigy shifted the
interest of neurologists from phenytoin as a treatment for trigeminal neuralgia
to carbamazepine, without sound scientific evidence. To date, no convincing
randomized controlled trials (RCTs) have been published supporting the role of
carbamazepine in trigeminal neuralgia, and we could not identify a single study
comparing the effects of phenytoin with those of carbamazepine. Accordingly,
phenytoin should probably be considered more often as a viable therapy for
(treatmentresistant) trigeminal neuralgia.