109451
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

New PD-L1 inhibitors in non-small cell lung cancer – impact of atezolizumab
Authors Seetharamu N, Preeshagul IR, Sullivan KM
Received 7 April 2017
Accepted for publication 6 June 2017
Published 13 July 2017 Volume 2017:8 Pages 67—78
DOI https://doi.org/10.2147/LCTT.S113177
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Akshita Wason
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Pan-Chyr Yang
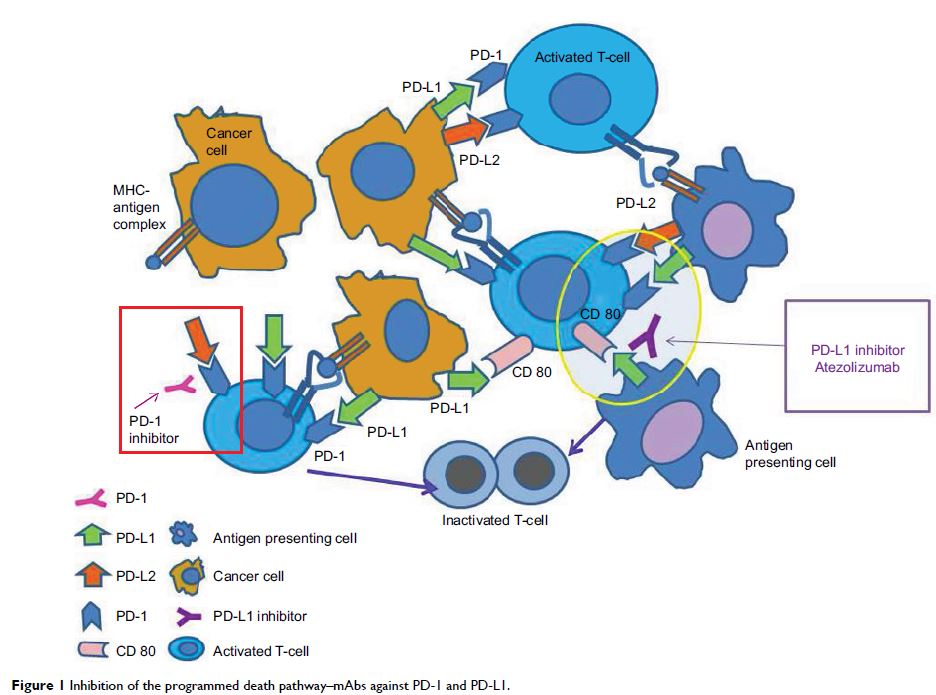
Abstract: The era of immunotherapy has changed the face of how we approach
treatment for many oncologic and hematologic malignancies. Lung cancer has been
in the forefront of checkpoint inhibition for the past 2 years and has paved
the path for other subspecialties. While PD-1 inhibitors nivolumab and
pembrolizumab have been approved for non-small cell lung cancer (NSCLC), this
review focuses on atezolizumab, its landmark studies, and ongoing trials.
Atezolizumab is the first programmed death ligand 1 (PD-L1) inhibitor to
receive US Food and Drug Administration (FDA) approval for metastatic NSCLC
patients who have progressed on frontline chemotherapy. This approval was based
on two open-label Phase II multicenter trials, POPLAR (NCT01903993) and BIRCH
(NCT02031458). Both studies revealed a benefit in overall survival (OS),
progression-free survival, and response rate in the atezolizumab arm when
compared to single-agent docetaxol. There were also fewest Grade 3–5
treatment-related adverse events (TRAEs) in the atezolizumab cohort. The
open-label randomized Phase III OAK trial (NCT02008227) further established the
role of atezolizumab in previously treated NSCLC. This study compared
atezolizumab with docetaxel in patients with advanced NSCLC (squamous or
nonsquamous histologies) who had progressed on one to two prior chemotherapy
regimens. OS in the PD-L1-enriched population was superior in the atezolizumab
arm (n=241) at 15.7 months compared with docetaxel (n=222) at 10.3 months
(hazard ratio [HR] 0.74, 95% confidence interval [CI] 0.58–0.93; p =0.0102). Patients lacking PD-L1
also had survival benefit with atezolizumab with a median OS (mOS) of 12.6
months versus 8.9 months with chemotherapy (HR 0.75, 95% CI 0.59–0.96). Benefit
was noted in both squamous and nonsquamous NSCLC subsets and regardless of
PD-L1 expressivity. As seen in the POPLAR and BIRCH studies, the toxicity
profile was significantly better with immunotherapy. The future is unfolding
rapidly as new checkpoint inhibitors are gaining FDA approval. It is still not
known if these agents will be used in combination with chemotherapy, with other
immune-modulating agents, radiation therapy, or all of the above. The results
of these studies investigating their use in combination with chemotherapy
agents, with other immunotherapy agents such as CTLA-4 inhibitors, and with
radiation therapy, are eagerly awaited.
Keywords: PD-1, PD-L1,
ADCC, CDC,checkpoint inhibition