109451
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Efficacy of flurbiprofen 8.75 mg delivered as a spray or lozenge in patients with sore throat due to upper respiratory tract infection: a randomized, non-inferiority trial in the Russian Federation
Authors Radkova E, Burova N, Bychkova V, DeVito R
Received 25 February 2017
Accepted for publication 17 May 2017
Published 6 July 2017 Volume 2017:10 Pages 1591—1600
DOI https://doi.org/10.2147/JPR.S135602
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 2
Editor who approved publication: Dr E. Alfonso Romero-Sandoval
Objective: To
assess the efficacy of flurbiprofen 8.75 mg delivered as a spray or
lozenge in patients with sore throat due to upper respiratory tract infection
(URTI).
Materials and
methods: This multicenter, double-blind,
double-dummy, non-inferiority study randomized 440 adults with recent-onset,
moderate-to-severe sore throat due to URTI to a single dose of either
flurbiprofen 8.75 mg spray (n=218) or flurbiprofen 8.75 mg lozenge
(n=222). The presence or absence of beta-hemolytic streptococci (A or C) was
confirmed by culture tests (throat swab). The primary efficacy end point was
the difference from baseline to 2 hours post-dose in sore throat pain intensity
scale (STPIS pain intensity difference [PID] 2h), a validated 100 mm
visual analog scale (from 0=“no pain” to 100=“severe pain”), with a
non-inferiority margin of −6 mm. Secondary end points included STPIS PID at
1 hour
(STPIS PID 1h) and over 2 hours (STPIS sum of sore throat pain intensity
differences [SPID]0–2h) and ratings of patient
satisfaction and investigator assessment of drug efficacy at 2 hours.
Safety (adverse events [AEs]) was also assessed.
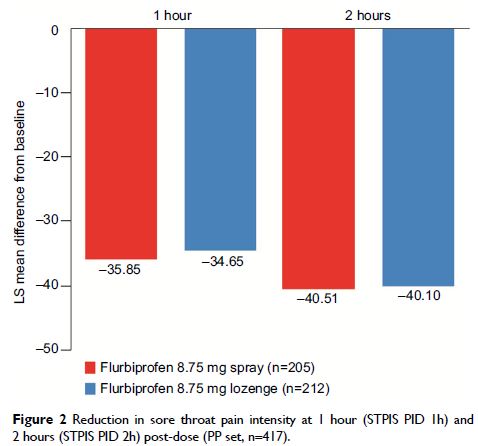
Results: Reductions
in sore throat pain intensity at 2 hours (STPIS PID 2h) were similar for
spray (least square mean −40.51) and lozenge (−40.10)
(difference: 0.41, 95% confidence interval [95% CI] −3.20,
4.01), with non-inferiority demonstrated. Subgroup analyses showed similar
efficacy (STPIS PID 2h) for patients testing positive or negative for Strep A
or C. There was no significant difference between spray and lozenge in STPIS
PID 1h or STPIS SPID0–2h, and patient satisfaction and
investigators’ assessment of efficacy at 2 hours were similar for both
groups. There were no significant differences in AEs between the two groups,
with 17 drug-related events across both groups, all being mild and none being
serious.
Conclusion: Both
formulations demonstrated comparable efficacy and safety profiles and provide
patients with two different treatment formats to choose from for effective
symptomatic relief of sore throat, depending on their preference.
Keywords: flurbiprofen,
non-inferiority, spray, lozenge