109568
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

对乙炔雌二醇/孕甾体 (Ethinylestradio/gestodene) 经皮避孕贴剂联合使用的药代动力学和安全性开放标签的 2 期比较研究
Authors Zhang C, Li H, Xiong X, Zhai S, Wei Y, Zhang S, Zhang Y, Xu L, Liu L
Received 26 December 2016
Accepted for publication 15 February 2017
Published 10 March 2017 Volume 2017:11 Pages 725—731
DOI https://doi.org/10.2147/DDDT.S131123
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Palas Chanda
Peer reviewer comments 3
Editor who approved publication: Dr Anastasios Lymperopoulos
Abstract: We investigated the pharmacokinetics and safety profiles of a newly
developed combined ethinylestradiol (EE)/gestodene (GSD) transdermal
contraceptive patch after a single-dose administration and compared with the
market available tablet formulation in healthy adult subjects. An open-label,
two-period comparative study was conducted in 12 healthy women volunteers. A
single dose of the study combined EE/GE transdermal contraceptive patch and
oral tablet (Milunet®) were administered. Blood
samples at different time points after dose were collected, and concentrations
were analyzed. A reliable, highly sensitive and accurate high-performance
liquid chromatography coupled with tandem mass spectrometry (HPLC/MS/MS) assay
method was developed in this study to determine the plasma concentrations of EE
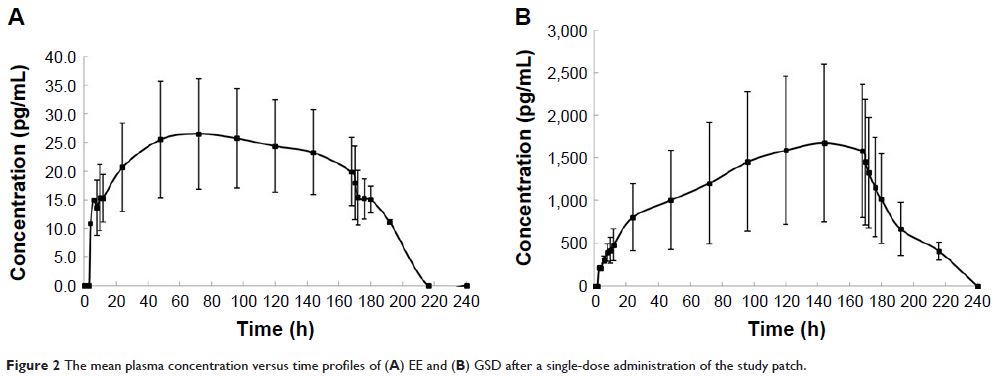
and GSD. Compared to the tablet, the study patch had a significantly decreased
maximum plasma concentration (C max), extended
time to reach the C max and half-life, as well as increased
clearance and apparent volume of distribution. The half-lives of EE and GSD of
the patch were 3.3 and 2.2 times, respectively, than the half-life of the
tablet. The areas under the plasma concentration–time curve (AUCs) of EE and
GSD of the patch were 8.0 and 16.2 times, respectively, than the AUC of the
tablet. No severe adverse event was observed during the whole study, and the
general safety was acceptable. In conclusion, compared to the oral tablet
Milunet, the study contraceptive patch was well tolerated and showed potent
drug exposure, significant extended half-life and stable drug concentrations.
Keywords: pharmacokinetics, safety,
ethinylestradiol/gestodene, transdermal contraceptive patch