109568
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

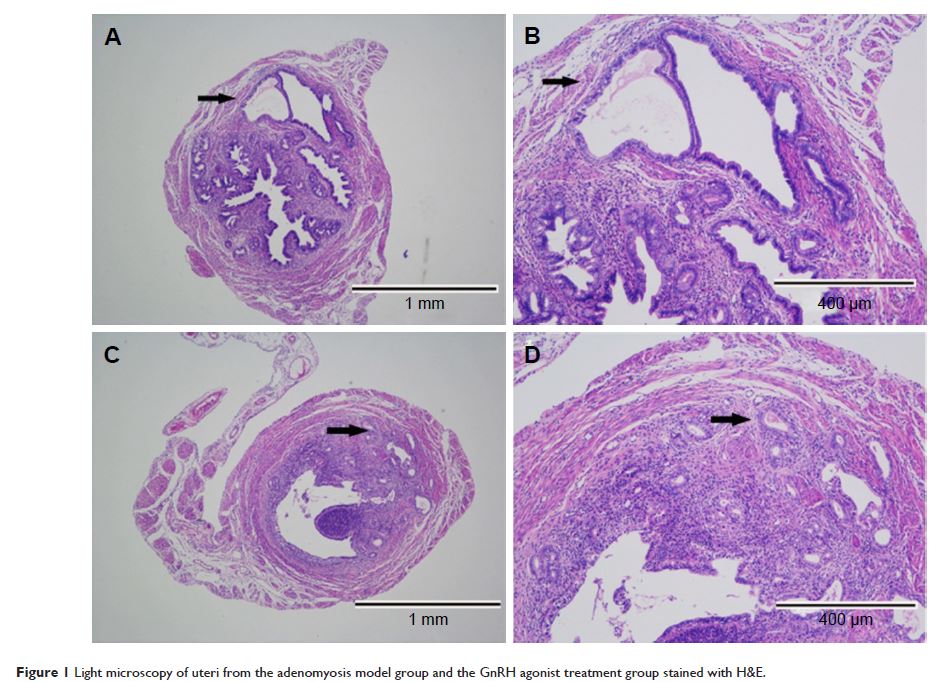
子宫腺肌病小鼠模型接受 GnRH 激动剂治疗后子宫内膜组织的转录组分析
Authors Guo S, Lu X, Gu R, Zhang D, Sun Y, Feng Y
Received 16 November 2016
Accepted for publication 2 February 2017
Published 9 March 2017 Volume 2017:11 Pages 695—704
DOI https://doi.org/10.2147/DDDT.S127889
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Chiung-Kuei Huang
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Purpose: Adenomyosis is a common, benign gynecological condition of the female reproductive
tract characterized by heavy menstrual bleeding and dysmenorrhea.
Gonadotropin-releasing hormone (GnRH) agonists are one of the medications used
in adenomyosis treatment; however, their underlying mechanisms are poorly
understood. Moreover, it is difficult to obtain endometrial samples from women
undergoing such treatment. To overcome this, we generated an adenomyosis mouse
model, which we treated with an GnRH agonist to determine its effect on
pregnancy outcomes. We also analyzed endometrial gene expression following GnRH
agonist treatment to determine the mechanisms that may affect pregnancy outcome
in individuals with adenomyosis.
Methods: Neonatal female mice were divided into a control group, an untreated
adenomyosis group, and an adenomyosis group treated with a GnRH agonist (n=6
each). The pregnancy outcome was observed and compared among the groups. Then,
three randomly chosen transcriptomes from endometrial tissues from day 4 of
pregnancy were analyzed between the adenomyosis group and the GnRH agonist
treatment group by RNA sequencing and quantitative reverse transcription
polymerase chain reaction (PCR).
Results: The litter size was significantly smaller in the adenomyosis group than
in the control group (7±0.28 vs 11±0.26; P <0.05). However, the average
live litter size was increased (10±0.28 vs 7±0.28; P <0.05) after
GnRH agonist treatment. Three hundred and fifty-nine genes were differentially
expressed in the GnRH agonist-treated group compared with the untreated group
(218 were downregulated and 141 were upregulated). Differentially expressed
genes were related to diverse biological processes, including estrogen
metabolism, cell cycle, and metabolite biosynthesis.
Conclusion: GnRH agonist treatment appears to improve the pregnancy outcome of
adenomyosis in a mouse model. Besides pituitary down-regulation, other possible
mechanisms such as the regulation of cell proliferation may play a role in
this. These new insights into GnRH agonist mechanisms will be useful for future
adenomyosis treatment.
Keywords: adenomyosis, GnRH agonist, mouse, RNA-seq, pregnancy outcome