109568
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

研究丝素蛋白纳米颗粒修饰的聚 (L-乳酸) 复合支架用于成骨细胞生长和分化
Authors Chen BQ, Kankala RK, Chen AZ, Yang DZ, Cheng XX, Jiang NN, Zhu K, Wang SB
Received 6 December 2016
Accepted for publication 1 February 2017
Published 8 March 2017 Volume 2017:12 Pages 1877—1890
DOI https://doi.org/10.2147/IJN.S129526
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Thiruganesh Ramasamy
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Abstract: Attempts to reflect the physiology of organs is quite an intricacy
during the tissue engineering process. An ideal scaffold and its surface
topography can address and manipulate the cell behavior during the regeneration
of targeted tissue, affecting the cell growth and differentiation
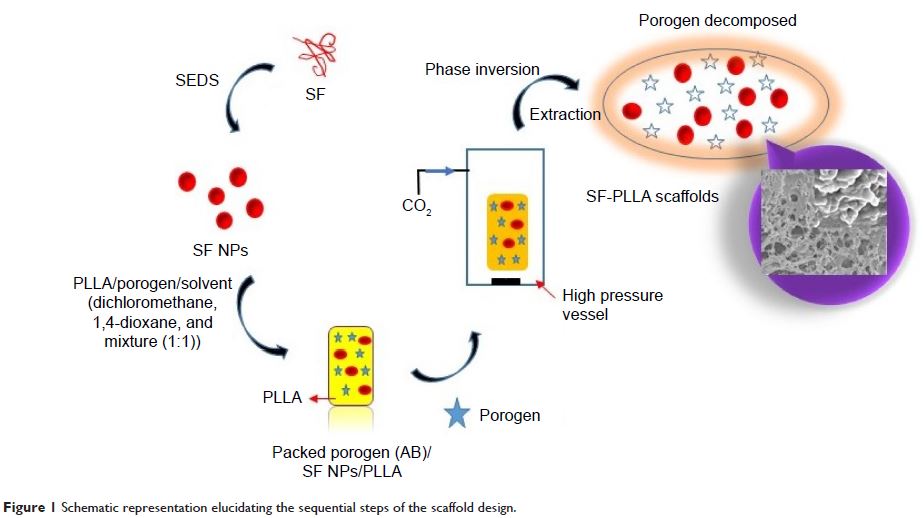
significantly. Herein, silk fibroin (SF) nanoparticles were incorporated into
poly(L-lactic acid) (PLLA) to prepare composite scaffolds via phase-inversion
technique using supercritical carbon dioxide (SC-CO2).
The SF nanoparticle core increased the surface roughness and hydrophilicity of
the PLLA scaffolds, leading to a high affinity for albumin attachment. The in
vitro cytotoxicity test of SF/PLLA scaffolds in L929 mouse fibroblast cells
indicated good biocompatibility. Then, the in vitro interplay between mouse
preosteoblast cell (MC3T3-E1) and various topological structures and
biochemical cues were evaluated. The cell adhesion, proliferation, osteogenic
differentiation and their relationship with the structures as well as SF
content were explored. The SF/PLLA weight ratio (2:8) significantly affected
the MC3T3-E1 cells by improving the expression of key players in the regulation
of bone formation, ie, alkaline phosphatase (ALP), osteocalcin (OC) and
collagen 1 (COL-1). These results suggest not only the importance of surface
topography and biochemical cues but also the potential of applying SF/PLLA
composite scaffolds as biomaterials in bone tissue engineering.
Keywords: super critical fluids, surface
topography, bone engineering, cellular adhesion, alkaline phosphatase