109568
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

利福平 - 利奈唑胺 (Rifapentine-linezolid) 加载的 PLGA 微球用于空洞型肺结核的介入治疗:制备和体外表征
Authors Huang J, Chen Z, Li Y, Li L, Zhang G
Received 16 November 2016
Accepted for publication 21 January 2017
Published 3 March 2017 Volume 2017:11 Pages 585—592
DOI https://doi.org/10.2147/DDDT.S127897
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Rammohan Devulapally
Peer reviewer comments 2
Editor who approved publication: Professor Jianbo Sun
Abstract: In this study, we aimed to design controlled-release microspheres for
the treatment of cavitary pulmonary tuberculosis (TB) for solving the issues of
poor drug delivery and short duration maintained at effective drug concentration
during bronchoscopic interventional therapy. We fabricated
rifapentine-linezolid-loaded poly(lactic acid-co-glycolic acid) microspheres
(RLPMs) using the oil-in-water emulsion solvent evaporation method and assessed
their in vitro release as well as the bronchial mucosal retention
characteristics. The microspheres are spherical in shape with a circular
concave on the surface. The particle size of RLPMs was 27.38±1.28 µm. The drug
loading of rifapentine and linezolid was 18.51±0.26 and 8.42%±0.24%,
respectively, while the encapsulation efficiencies were 55.53±0.78 and
16.87%±0.47%, respectively (n=3). During the burst release phase of the in
vitro release test, 21.37%±0.68% rifapentine was released in 3 days and
43.56%±2.54% linezolid was released in 1 day. Then, both the drugs entered the
sustained release phase. Finally, the cumulative percentage release of
rifapentine and linezolid in 14 days was 27.61±1.52 and 51.01%±3.31%,
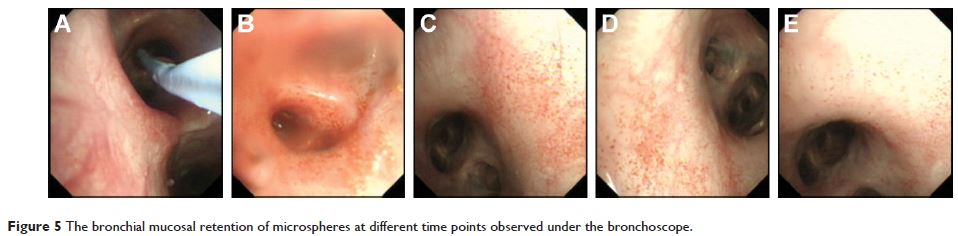
respectively (n=3). Bronchoscopic observation revealed that the controlled-release
microspheres could slowly release the drugs and retain them on the surface of
bronchial mucosa of canines for 20 days. These results indicated that the
fabricated microspheres exhibited a significant sustained release effect and
could effectively retain the drugs on the surface of bronchial mucosa.
Therefore, this study provides a theoretical and practical foundation for the
development of fabricated microspheres loaded with multiple anti-TB drugs in
the bronchoscopic interventional therapy of cavity pulmonary TB.
Keywords: rifapentine, linezolid,
poly(lactic-co-glycolic acid), controlled-release microspheres, bronchoscopy,
cavity pulmonary tuberculosis