109568
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

用于复发性卵巢癌患者化疗的抗血管生成药物:一项综合分析
Authors Yi SY, Zeng LJ, Kuang Y, Cao ZJ, Zheng CJ, Zhang Y, Liao M, Yang L
Received 16 August 2016
Accepted for publication 5 November 2016
Published 17 February 2017 Volume 2017:10 Pages 973—984
DOI https://doi.org/10.2147/OTT.S119879
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Akshita Wason
Peer reviewer comments 3
Editor who approved publication: Dr Samir Farghaly
Objective: The value of antiangiogenic inhibitors for patients with recurrent
ovarian cancer has not been completely affirmed. Therefore, we aimed to assess
the effectiveness and toxicities of various antiangiogenic drugs for the
treatment of recurrent ovarian cancer.
Methods: In this meta-analysis, we searched PubMed, EMBASE, and the Cochrane
Central Register of Controlled Trials databases for complete randomized
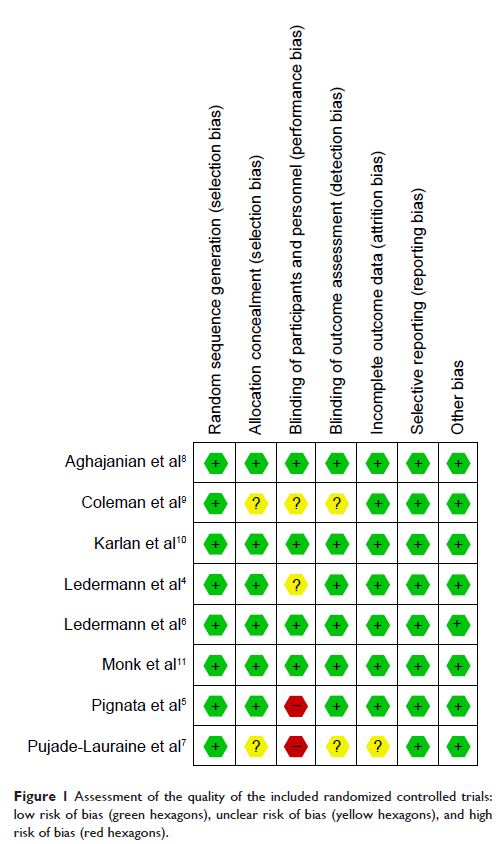
controlled trials. The searches were extended to May 15, 2016. The risk of bias
of the included studies was evaluated via a Cochrane systematic evaluation, and
the statistical analyses were performed using RevMan 5.2 software.
Results: In total, we included 8 randomized controlled trials involving 3,211
patients and divided them into 3 groups, vascular endothelial growth factor
receptor inhibitors (VEGFRIs), vascular endothelial growth factor (VEGF)
inhibitors (bevacizumab), and angiopoietin inhibitors (trebananib). The
progression-free survival improved significantly in all the groups being given
antiangiogenic drugs (hazard ratio [HR]: 0.55, 95% confidence interval [CI]:
0.45–0.67, I 2=0%, P <0.00001 for the VEGFRI group;
HR: 0.53, 95% CI: 0.45–0.63, I 2=51%, P <0.00001 for the VEGF
inhibitor group; HR: 0.67, 95% CI: 0.58–0.77, I 2=0%, P <0.00001 for the trebananib
group). Overall survival was obviously prolonged in the VEGFRI (HR: 0.76, 95%
CI: 0.59–0.97, I 2=0%, P =0.03), the VEGF inhibitor (HR:
0.87, 95% CI: 0.77–0.99, I 2=0%, P =0.03), and trebananib groups
(HR: 0.81, 95% CI: 0.67–0.99, I 2=0%, P =0.04). The incidence of grade
3/4 side effects was different among the 3 groups, for example, proteinuria,
hypertension, gastrointestinal perforation, and arterial thromboembolism were
presented in the VEGF inhibitor group. Increased incidences of fatigue,
diarrhea, and hypertension were seen in the VEGFRI group, and the trebananib
group had a higher incidence of hypokalemia.
Conclusion: This meta-analysis showed that antiangiogenic drugs improved the
progression-free survival. The VEGFRI, bevacizumab, and trebananib groups
showed increased overall survival. Adding antiangiogenic drugs to chemotherapy
treatment resulted in a higher incidence of grade 3/4 side effects, but these
were manageable.
Keywords: antiangiogenesis, recurrent ovarian cancer, progression-free survival,
overall survival, toxicity