109568
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

天冬酰胺酰内肽酶通过对 EGFR/JNK/ERK 信号通路起调节作用的 IQGAP1 来改善胃癌中微管靶向药物的抗性
Authors Cui Y, Li Q, Li H, Wang Y, Wang H, Chen W, Zhang S, Cao J, Liu T
Received 24 October 2016
Accepted for publication 27 December 2016
Published 3 February 2017 Volume 2017:10 Pages 627—643
DOI https://doi.org/10.2147/OTT.S125579
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Akshita Wason
Peer reviewer comments 2
Editor who approved publication: Dr Chiung-Kuei Huang
Purpose: In recent years, understanding of the role of asparaginyl endopeptidase
(AEP) in tumorigenesis has steadily increased. In this study, we investigated
whether AEP expression correlates with sensitivity to chemotherapeutic drugs in
gastric cancer and explored the mechanism.
Patients and
methods: AEP expression in the serum of patients’ peripheral blood was measured
by enzyme-linked immunosorbent assay. Patient survival time was evaluated using
univariate and multivariate analyses. Mass spectrometry and
co-immunoprecipitation assays were utilized to discover proteins that interact
with AEP. Gastric cancer cell lines were established, in which AEP was
overexpressed or knocked out using lentiviral CRISPR. The proliferative
abilities of these cell lines in response to chemotherapy agents were evaluated
using the Cell Counting Kit-8 method. Gene expression changes in these lines
were assessed by real-time polymerase chain reaction and Western blot.
Results: Patients with low expression of AEP were significantly more likely to
have a good prognosis and experience complete response or partial response
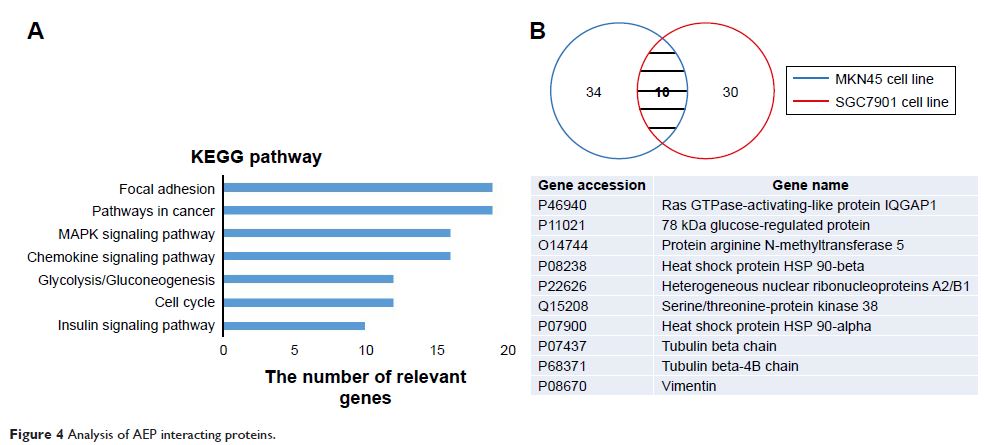
after treatment with docetaxel/S-1 regimen. Mass spectrum analysis showed that
several proteins in the focal adhesion and mitogen-activated protein kinase
signaling pathways interacted with AEP. IQGAP1 was confirmed to be one of the
proteins interacting with AEP, and its protein level increased when AEP was
knocked out. AEP knockout decreased resistance to microtubule inhibitors,
including paclitaxel, docetaxel, and T-DM1. The expression levels of MDR1,
p-EGFR, p-JNK, p-ERK, and p-Rac1/cdc42 were decreased in AEP knockout gastric
cancer cell lines, and inhibitors of both JNK and ERK could block AEP-induced
expression of MDR1.
Conclusion: AEP was not only a prognostic factor but also a predictive marker. AEP
knockout could inhibit the activity of the EGFR/JNK/ERK signaling pathway and
improve sensitivity to microtubule inhibitors through interacting with IQGAP1.
Keywords: Asparaginyl endopeptidase, MAP kinase signaling pathway, drug
resistance, stomach cancer