109568
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Drugs in development for toxoplasmosis: advances, challenges, and current status
Authors Alday PH, Doggett JS
Received 6 July 2016
Accepted for publication 23 November 2016
Published 25 January 2017 Volume 2017:11 Pages 273—293
DOI https://doi.org/10.2147/DDDT.S60973
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Dragan Hrncic
Peer reviewer comments 3
Editor who approved publication: Dr James Janetka
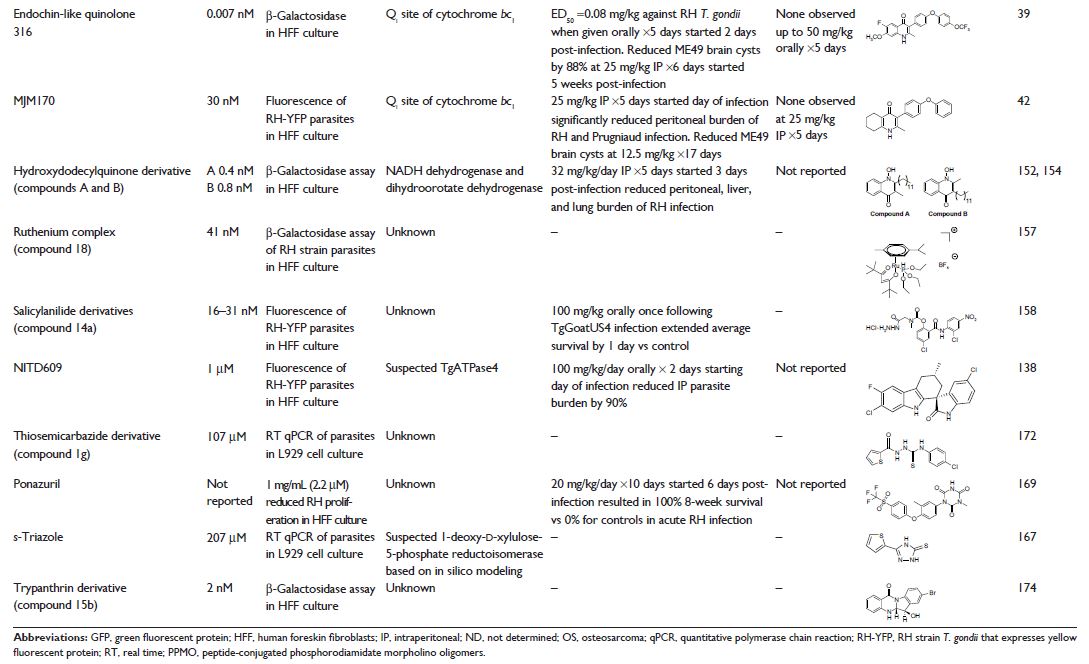
Abstract: Toxoplasma
gondii causes fatal and debilitating brain and eye diseases. Medicines that are
currently used to treat toxoplasmosis commonly have toxic side effects and require
prolonged courses that range from weeks to more than a year. The need for long
treatment durations and the risk of relapsing disease are in part due to the
lack of efficacy against T. gondii tissue
cysts. The challenges for developing a more effective treatment for
toxoplasmosis include decreasing toxicity, achieving therapeutic concentrations
in the brain and eye, shortening duration, eliminating tissue cysts from the
host, safety in pregnancy, and creating a formulation that is inexpensive and practical
for use in resource-poor areas of the world. Over the last decade, significant
progress has been made in identifying and developing new compounds for the
treatment of toxoplasmosis. Unlike clinically used medicines that were
repurposed for toxoplasmosis, these compounds have been optimized for efficacy
against toxoplasmosis during preclinical development. Medicines with enhanced
efficacy as well as features that address the unique aspects of toxoplasmosis
have the potential to greatly improve toxoplasmosis therapy. This review
discusses the facets of toxoplasmosis that are pertinent to drug design and the
advances, challenges, and current status of preclinical drug research for
toxoplasmosis.
Keywords: Toxoplasma gondii , therapeutics,
preclinical medicine, experimental medicine, mechanism of action, Apicomplexa