109568
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

阿帕替尼 (Apatinib) 用于 EGFR 野生型和 ALK 阴性晚期肺腺癌的后二线治疗
Authors Fang S, Zhang H, Zhang Y, Xie W
Received 4 November 2016
Accepted for publication 8 December 2016
Published 18 January 2017 Volume 2017:10 Pages 447—452
DOI https://doi.org/10.2147/OTT.S126613
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Ru Chen
Peer reviewer comments 3
Editor who approved publication: Dr Yao Dai
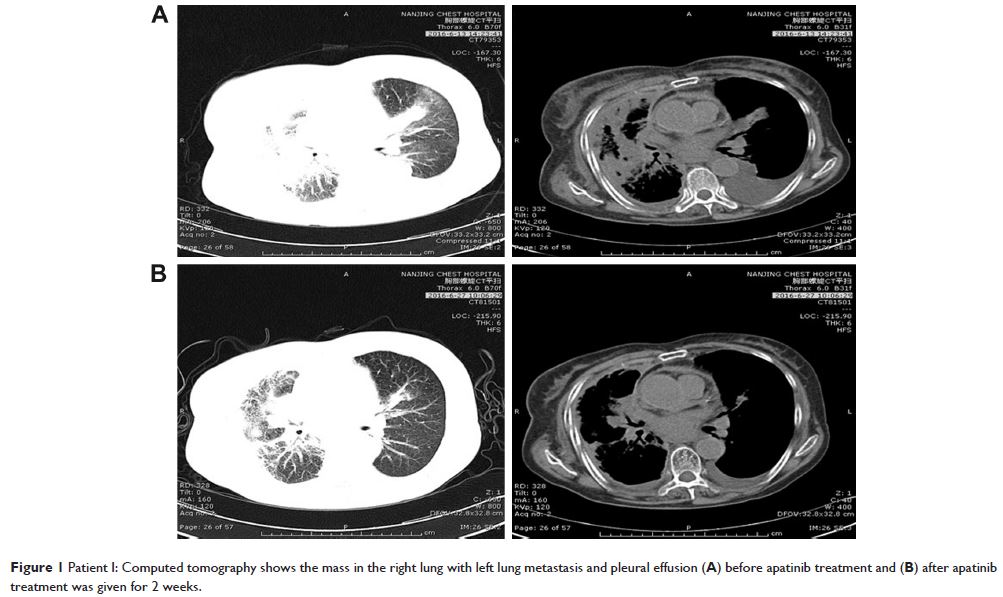
Abstract: In the absence of a driver mutation, chemotherapy is the standard
treatment option as first- and second-line therapy for advanced non-small-cell
lung cancer (NSCLC). Though a large number of patients are suitable for post
second-line therapies, the quality and quantity of the available drugs in this
setting is poor. Apatinib, a small molecule vascular endothelial growth factor
receptor-2 (VEGFR-2) tyrosine kinase inhibitor, is a first-generation oral
antiangiogenesis drug approved in the People’s Republic of China for use as a
subsequent line of treatment for advanced gastric cancer. Herein, we report
three cases of advanced NSCLC with epidermal growth factor receptor wild-type
and anaplastic lymphoma kinase-negative status, wherein the patients showed
partial response to apatinib. Moreover, the three patients have achieved a
progression-free survival of 2.8, 5.8, and 6 months, respectively. The main
toxicities were hypertension, proteinuria, and hand–foot syndrome. Apatinib may
provide an additional option for the treatment of advanced NSCLC, especially
for advanced lung adenocarcinoma without a driver mutation.
Keywords: non-small-cell
lung cancer, angiogenesis, apatinib, VEGFR-2