109568
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

新型的抗微生物肽修饰的阿奇霉素 (Azithromycin) 脂质体对抗耐甲氧西林金黄色葡萄球菌 (Staphylococcus aureus )
Authors Liu X, Li Z, Wang X, Chen Y, Wu F, Men K, Xu T, Luo Y, Yang L
Received 24 February 2016
Accepted for publication 27 May 2016
Published 14 December 2016 Volume 2016:11 Pages 6781—6794
DOI https://doi.org/10.2147/IJN.S107107
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Professor Farooq Shiekh
Peer reviewer comments 2
Editor who approved publication: Dr Lei Yang
Abstract: Infections caused by multidrug-resistant bacteria, such as
methicillin-resistant Staphylococcus aureus (MRSA),
have become a public threat; therefore, development of new antimicrobial drugs
or strategies is urgently required. In this study, a new antibacterial peptide
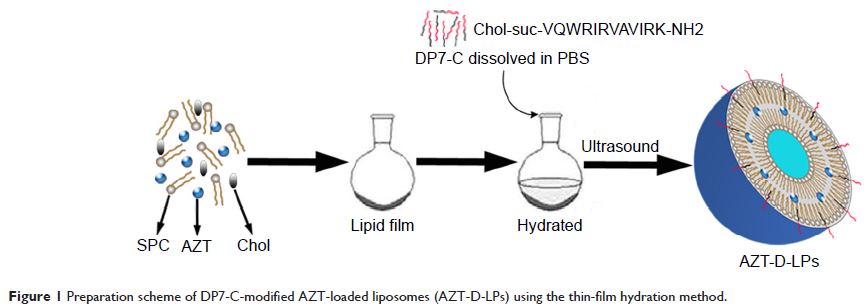
DP7-C (Chol-suc-VQWRIRVAVIRK-NH2) and DP7-C-modified azithromycin (AZT)-loaded
liposomes (LPs) are developed for the treatment of MRSA infection, and it was
found that DP7-C inserted into the LP lipid bilayer not only functioned as a
carrier to encapsulate the antibiotic AZT but also synergized the antibacterial
effect of the encapsulated AZT. In vitro assays showed that DP7-C-modified LPs
possessed sustained drug release profile and immune regulatory effect and did
not show obvious cytotoxicity in mammal cells, but they did not possess direct antibacterial
activity in vitro. In vivo studies revealed that DP7-C-modified LPs did not
exhibit obvious side effects or toxicity in mice but were able to significantly
reduce the bacterial counts in an MRSA-infectious mouse model and possessed
high antibacterial activity. In particular, DP7-C-modified AZT-loaded LPs
showed more positive therapeutic effects than either DP7-C-modified blank LPs
or nonmodified AZT-loaded LPs treatment alone. Molecular mechanism studies
demonstrated that DP7-C formulations effectively upregulated the production of
anti-inflammatory cytokines and chemokines without inducing harmful immune
response, suggesting that DP7-C was synergistic with AZT against the bacterial
infection by activating the innate immune response. Most importantly, although
DP7-C activated the innate immune response, it did not possess direct
antibacterial activity in vitro, indicating that DP7-C did not possess the
potential to induce bacteria resistance. The findings indicate that
DP7-C-modified AZT-loaded LPs developed in this study have a great potential
required for the clinical treatment of MRSA infections.
Keywords: DP7-C, azithromycin, antimicrobial
resistance, MRSA infections, immune-regulation