109568
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Baicalein 在大鼠中改善阿尔茨海默病诱导的行为功能障碍
Authors Zhou L, Tan S, Shan YL, Wang YG, Cai W, Huang XH, Liao XY, Li HY, Zhang L, Zhang BJ, Lu ZQ
Received 18 July 2016
Accepted for publication 25 August 2016
Published 9 December 2016 Volume 2016:12 Pages 3145—3152
DOI https://doi.org/10.2147/NDT.S117469
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Professor Wai Kwong Tang
Background: Alzheimer’s
disease (AD) is considered to be a neurodegenerative disorder that is
characterized by increased oxidative stress. Medicinal plants, with their
antioxidant properties, have been used to cure several human diseases. The aim
of the current study was to explore the protective and therapeutic effect of
baicalein on AD-induced rats.
Materials and methods: Swiss Wistar rats were used in the study. The rats
were divided into five groups. Group I: normal control group treated with
water; Group II: disease control treated with AlCl3 to induce the mimicking AD for 4
successive weeks (SW); Group III: normal control group treated with baicalein
(5 mg/kg) for 2 SW followed by combination of baicalein and AlCl3 for 4 SW; Group IV: normal control group treated
with baicalein (10 mg/kg) for 2 SW followed by combination of baicalein and
AlCl3 for 4
SW; Group V: normal control group treated with rivastigmine (0.3 mg/kg) for 2
SW followed by combination of rivastigmine and AlCl3 for 4 SW. Moreover, the therapeutic groups
are as follows: Group VI: AD disease control treated with AlCl3 for 4
SW and serving as the therapeutic positive group; Group VII: AD disease control
+ baicalein (5 mg/kg) for 12 SW; Group VIII: AD disease control + baicalein (10
mg/kg) for 12 SW; Group IX: AD disease control + rivastigmine (0.3 mg/kg) for
12 SW. Behavioral test, T-maze, and rotarod test were also performed before and
after the treatment. At the end of the experimental study, all the rats were
sacrificed and their brains were removed and divided into two portions. The
first portion was homogenated for estimating the level of acetylcholinesterase
(AchE) and acetylcholine (Ach). Another portion was used for histopathological
evaluation.
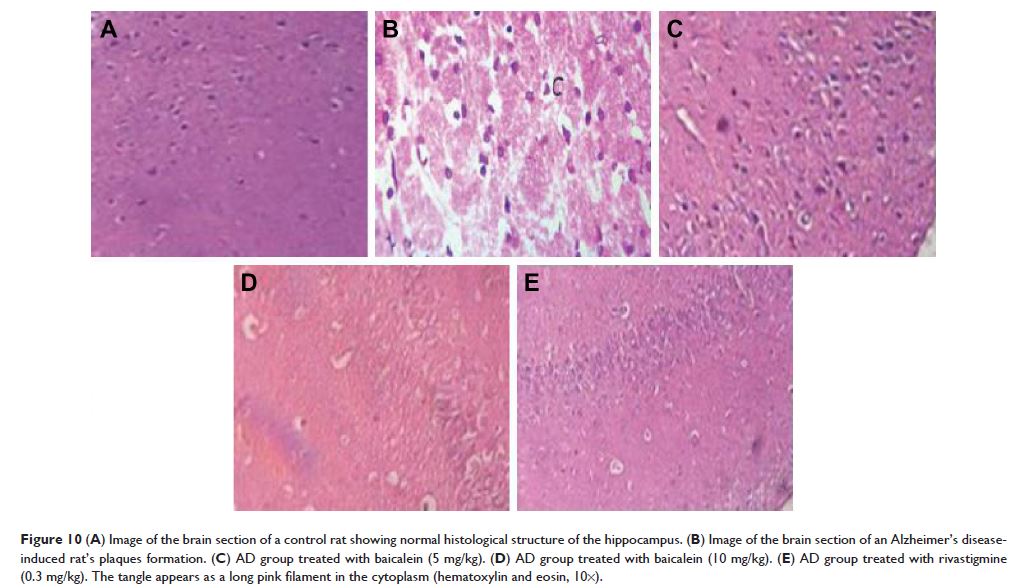
Results: The current investigation showed that baicalein
significantly reduced the duration of revolving on the rotarod, cage activity,
and T-maze activity in a dose-dependent manner compared with the AD control
group rats. It also altered the AchE and Ach levels in the brain homogenates.
The histopathology study also provides strength to the protective effect of
baicalein.
Conclusion: The current study showed that baicalein significantly
(P <0.05) improved the
biochemical and histopathological condition of AD in rats.
Keywords: baicalein, Alzheimer’s disease,
acetylcholinesterase, acetylcholine