108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
装载抗人死亡受体 5 的单链抗体的羟乙基壳聚糖纳米颗粒的制备及功能研究
Authors Yang J, Huang X, Luo F, Cheng X, Cheng L, Liu B, Chen L, Hu R, Shi C, Zhuang G, Yin P
Published Date May 2014 Volume 2014:7 Pages 779—787
DOI http://dx.doi.org/10.2147/OTT.S59872
Received 30 December 2013, Accepted 11 March 2014, Published 21 May 2014
Objective: To prepare hydroxyethyl chitosan nanoparticles loaded with anti-human death receptor 5 single-chain antibody, and study their characteristics, functions, and mechanisms of action.
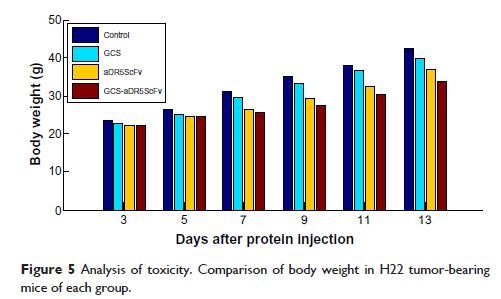
Materials and methods: The anti-human death receptor 5 single-chain antibody was constructed and expressed. Protein-loaded hydroxyethyl chitosan nanoparticles were prepared, and their size, morphology, particle-size distribution and surface zeta potential were measured by scanning electron microscopy and laser particle-size analysis. Mouse H22 hepatocellular carcinoma cells were cultured, and growth inhibition was examined using the CellTiter-Blue cell-viability assay. Flow cytometry and Hoechst 33342 were employed to measure cell apoptosis. Kunming mice with H22 tumor models were treated with protein-loaded hydroxyethyl chitosan nanoparticles, and their body weight and tumor size were measured, while hematoxylin and eosin staining was used to detect antitumor effects in vivo and side effects from tumors.
Results: The protein-loaded hydroxyethyl chitosan nanoparticles had good stability; the zeta potential was -24.2±0.205, and the dispersion index was 0.203. The inhibition of the protein-loaded hydroxyethyl chitosan nanoparticles on H22 growth was both time- and dose-dependent. Increased expressions of active caspase 8, active caspase 3, and BAX were detected following treatment. The average weight gain, tumor weight, and mean tumor volume of the protein and protein-loaded hydroxyethyl chitosan nanoparticle groups were significantly different (P <0.05) compared with the phosphate-buffered saline group.
Conclusion: The protein-loaded hydroxyethyl chitosan nanoparticles effectively suppressed tumor growth, indicating that nanotechnology has the potential for broad application in cancer therapy.
Keywords: anticancer effect, DR5, GCS-aDR5ScFv, H22
Materials and methods: The anti-human death receptor 5 single-chain antibody was constructed and expressed. Protein-loaded hydroxyethyl chitosan nanoparticles were prepared, and their size, morphology, particle-size distribution and surface zeta potential were measured by scanning electron microscopy and laser particle-size analysis. Mouse H22 hepatocellular carcinoma cells were cultured, and growth inhibition was examined using the CellTiter-Blue cell-viability assay. Flow cytometry and Hoechst 33342 were employed to measure cell apoptosis. Kunming mice with H22 tumor models were treated with protein-loaded hydroxyethyl chitosan nanoparticles, and their body weight and tumor size were measured, while hematoxylin and eosin staining was used to detect antitumor effects in vivo and side effects from tumors.
Results: The protein-loaded hydroxyethyl chitosan nanoparticles had good stability; the zeta potential was -24.2±0.205, and the dispersion index was 0.203. The inhibition of the protein-loaded hydroxyethyl chitosan nanoparticles on H22 growth was both time- and dose-dependent. Increased expressions of active caspase 8, active caspase 3, and BAX were detected following treatment. The average weight gain, tumor weight, and mean tumor volume of the protein and protein-loaded hydroxyethyl chitosan nanoparticle groups were significantly different (P <0.05) compared with the phosphate-buffered saline group.
Conclusion: The protein-loaded hydroxyethyl chitosan nanoparticles effectively suppressed tumor growth, indicating that nanotechnology has the potential for broad application in cancer therapy.
Keywords: anticancer effect, DR5, GCS-aDR5ScFv, H22