108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
伊马替尼耐药胃肠道间质瘤的第二代酪氨酸激酶抑制剂的临床疗效: 一项最近的临床试验荟萃分析
Authors Wu L, Zhang Z, Yao H, Liu K, Wen Y, Xiong L
Published Date October 2014 Volume 2014:8 Pages 2061—2067
DOI http://dx.doi.org/10.2147/DDDT.S63840
Received 10 March 2014, Accepted 17 April 2014, Published 30 October 2014
Background: Primary and secondary resistance to imatinib, a selective receptor tyrosine kinase inhibitor (TKI), is a serious clinical problem in the control of advanced gastrointestinal stromal tumors (GIST). Here we report on a meta-analysis we performed to evaluate the efficacy of second-generation TKIs in the treatment of patients with imatinib-resistant GIST.
Methods: Randomized controlled trials evaluating the clinical efficacy of second-generation TKIs were identified by searching PubMed and EMBASE from 2000 to February 2014. Outcomes subjected to analysis were progression-free survival and overall survival. Statistical analyses were performed using Review Manager version 5.1.0 (Cochrane Collaboration, Oxford, UK). Weighted hazard ratios (HR) with 95% confidence intervals (CIs) were calculated for the outcomes. Fixed-effects or random-effects models were used, depending on the degree of heterogeneity across the selected studies.
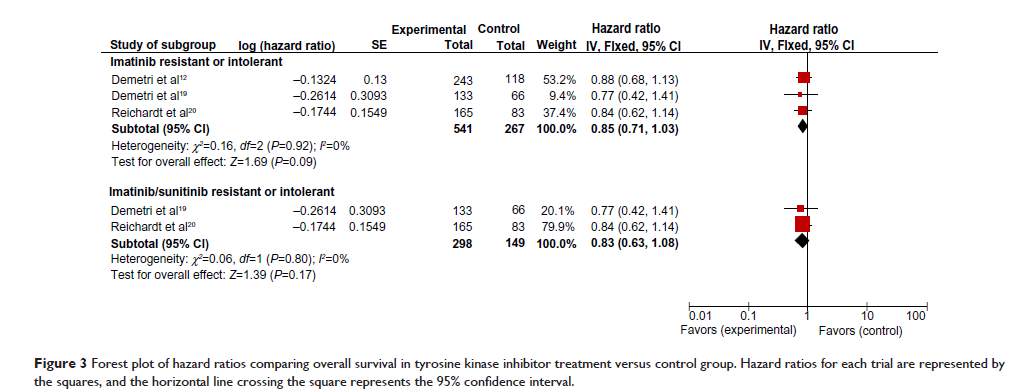
Results: Three randomized controlled trials were selected for meta-analysis. Among imatinib-resistant or imatinib-intolerant patients, 541 received second-generation TKIs (sunitinib, nilotinib, or regorafenib) and 267 controls received placebo or best supportive care. Progression-free survival was significantly improved in the TKI-treated group (HR 0.38; 95% CI 0.24–0.59; P <0.0001). No statistically significant difference was detected in overall survival between the treatment group and the control group (HR 0.85; 95% CI 0.71–1.03; P =0.09). In the subgroup of patients who were resistant or intolerant to both imatinib and sunitinib, TKI therapy (nilotinib or regorafenib) improved progression-free survival (HR 0.40; 95% CI 0.19–0.84; P =0.02) but not overall survival (HR 0.83; 95% CI 0.63–1.08; P =0.17). Regorafenib was shown to be effective in terms of progression-free survival across different subpopulations of patients who were resistant to both imatinib and sunitinib.
Conclusion: Second-generation TKIs (sunitinib, nilotinib, and regorafenib) are effective in improving progression-free survival but not overall survival in patients with GIST who are resistant or intolerant to imatinib or to imatinib and sunitinib. Regorafenib is promising as a third-line treatment option for patients with advanced GIST.
Keywords: tyrosine kinase inhibitor, gastrointestinal stromal tumor, imatinib, nilotinib, regorafenib
Methods: Randomized controlled trials evaluating the clinical efficacy of second-generation TKIs were identified by searching PubMed and EMBASE from 2000 to February 2014. Outcomes subjected to analysis were progression-free survival and overall survival. Statistical analyses were performed using Review Manager version 5.1.0 (Cochrane Collaboration, Oxford, UK). Weighted hazard ratios (HR) with 95% confidence intervals (CIs) were calculated for the outcomes. Fixed-effects or random-effects models were used, depending on the degree of heterogeneity across the selected studies.
Results: Three randomized controlled trials were selected for meta-analysis. Among imatinib-resistant or imatinib-intolerant patients, 541 received second-generation TKIs (sunitinib, nilotinib, or regorafenib) and 267 controls received placebo or best supportive care. Progression-free survival was significantly improved in the TKI-treated group (HR 0.38; 95% CI 0.24–0.59; P <0.0001). No statistically significant difference was detected in overall survival between the treatment group and the control group (HR 0.85; 95% CI 0.71–1.03; P =0.09). In the subgroup of patients who were resistant or intolerant to both imatinib and sunitinib, TKI therapy (nilotinib or regorafenib) improved progression-free survival (HR 0.40; 95% CI 0.19–0.84; P =0.02) but not overall survival (HR 0.83; 95% CI 0.63–1.08; P =0.17). Regorafenib was shown to be effective in terms of progression-free survival across different subpopulations of patients who were resistant to both imatinib and sunitinib.
Conclusion: Second-generation TKIs (sunitinib, nilotinib, and regorafenib) are effective in improving progression-free survival but not overall survival in patients with GIST who are resistant or intolerant to imatinib or to imatinib and sunitinib. Regorafenib is promising as a third-line treatment option for patients with advanced GIST.
Keywords: tyrosine kinase inhibitor, gastrointestinal stromal tumor, imatinib, nilotinib, regorafenib