109451
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
使用血管内皮生长因子受体酪氨酸激酶抑制剂治疗相关的死亡风险:对 41 项随机对照试验进行的一项荟萃分析
Authors Hong SD, Fang WF, Liang WH, Yan Y, Zhou T, Qin T, Wu X, Ma YX, Zhao YY, Yang YP, Hu ZH, Xue C, Hou X, Chen Y, Huang Y, Zhao HY, Zhang L
Published Date October 2014 Volume 2014:7 Pages 1851—1867
DOI http://dx.doi.org/10.2147/OTT.S68386
Received 25 May 2014, Accepted 16 July 2014, Published 7 October 2014
Background: Vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKIs) have widely been used in advanced cancer. However, these drugs may also lead to serious adverse events. The present meta-analysis aimed to determine the overall incidence and risk of deaths due to VEGFR-TKIs with more detailed subgroup analysis.
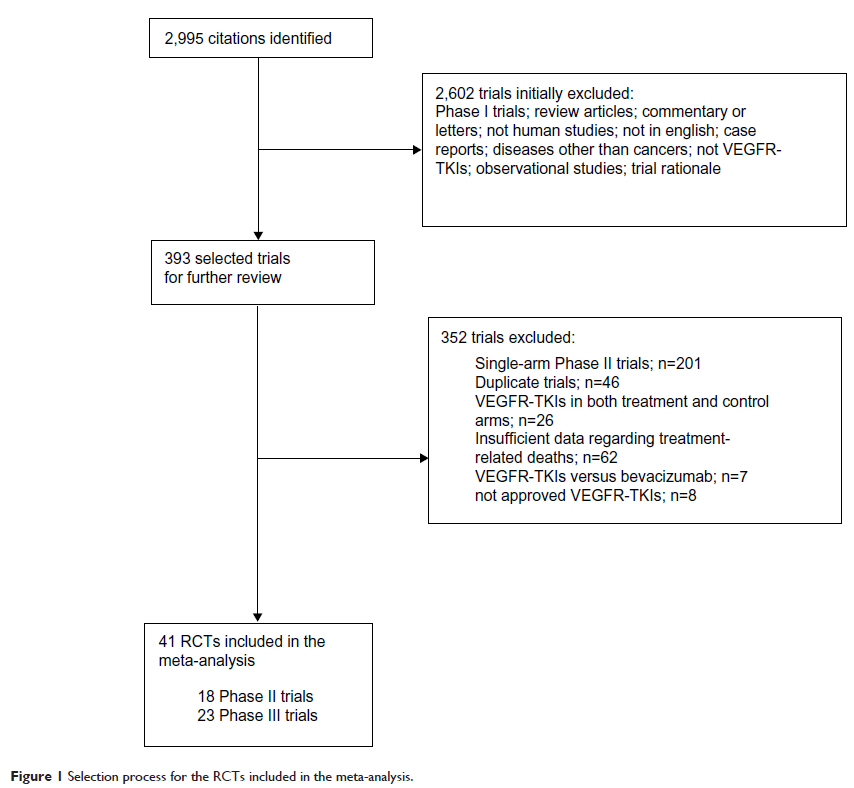
Materials and methods: PubMed, Web of Science, and Cochrane databases were searched for randomized controlled trials (RCTs) that compared VEGFR-TKIs with non-VEGFR-TKIs in the treatment of solid cancer. Pooled incidence, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using random-effects or fixed-effects models based on the heterogeneity of included trials.
Results: A total of 14,139 participants from 41 RCTs were enrolled. The pooled incidence of death due to VEGFR-TKIs was 1.9% (95% CI: 1.6%–2.3%) with an OR of 1.85 (95% CI: 1.33–2.58; P <0.01) when compared with control groups. On subgroup analysis, significantly increased risk of death was found in patients with nonsmall-cell lung cancer (OR: 2.37; 95% CI: 1.19–4.73; P =0.01) and colorectal cancer (OR: 2.84; 95% CI: 1.02–7.96; P =0.05). Among different VEGFR-TKIs, sorafenib and sunitinib had significant risk of death when compared with control arms, respectively. VEGFR-TKIs in combination with other antineoplastic agents, but not VEGFR-TKI monotherapy, significantly increased the risk of treatment-related deaths. No heterogeneity was noted across all the prespecified subgroups regarding ORs.
Conclusion: The present work pointed out a significantly increased risk of death due to VEGFR-TKIs. Close monitoring should be emphasized in patients receiving these drugs.
Keywords: cancer, tyrosine kinase inhibitors, treatment-related death, meta-analysis
Materials and methods: PubMed, Web of Science, and Cochrane databases were searched for randomized controlled trials (RCTs) that compared VEGFR-TKIs with non-VEGFR-TKIs in the treatment of solid cancer. Pooled incidence, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using random-effects or fixed-effects models based on the heterogeneity of included trials.
Results: A total of 14,139 participants from 41 RCTs were enrolled. The pooled incidence of death due to VEGFR-TKIs was 1.9% (95% CI: 1.6%–2.3%) with an OR of 1.85 (95% CI: 1.33–2.58; P <0.01) when compared with control groups. On subgroup analysis, significantly increased risk of death was found in patients with nonsmall-cell lung cancer (OR: 2.37; 95% CI: 1.19–4.73; P =0.01) and colorectal cancer (OR: 2.84; 95% CI: 1.02–7.96; P =0.05). Among different VEGFR-TKIs, sorafenib and sunitinib had significant risk of death when compared with control arms, respectively. VEGFR-TKIs in combination with other antineoplastic agents, but not VEGFR-TKI monotherapy, significantly increased the risk of treatment-related deaths. No heterogeneity was noted across all the prespecified subgroups regarding ORs.
Conclusion: The present work pointed out a significantly increased risk of death due to VEGFR-TKIs. Close monitoring should be emphasized in patients receiving these drugs.
Keywords: cancer, tyrosine kinase inhibitors, treatment-related death, meta-analysis