109451
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
复发性和转移性鼻咽癌患者在铂类为基础的化疗失败后,进行 S-1 化疗的安全与疗效:多机构回顾性分析
Authors Peng PJ, Cheng H, Ou XQ, Zeng LJ, Wu X, Liu YM, Lin Z, Tang YN, Wang SY, Zhang HY, Chen ZB
Published Date August 2014 Volume 2014:8 Pages 1083—1087
DOI http://dx.doi.org/10.2147/DDDT.S67592
Received 12 May 2014, Accepted 31 May 2014, Published 14 August 2014
Purpose: This retrospective study evaluates the efficacy and safety of S-1 chemotherapy for recurrent and metastatic nasopharyngeal carcinoma patients after failure of platinum-based chemotherapy.
Patients and methods: Thirty-nine patients with recurrent and metastatic nasopharyngeal carcinoma who failed previous platinum-based chemotherapy received oral S-1 chemotherapy (twice daily from day 1 to 14) every 3 weeks. The dose of S-1 was determined according to the body surface area (BSA): 40 mg twice a day for BSA <1.25 m2; 50 mg twice a day for 1.25 m2 ≤BSA<1.5 m2; and 60 mg twice a day for BSA ≥1.5 m2.
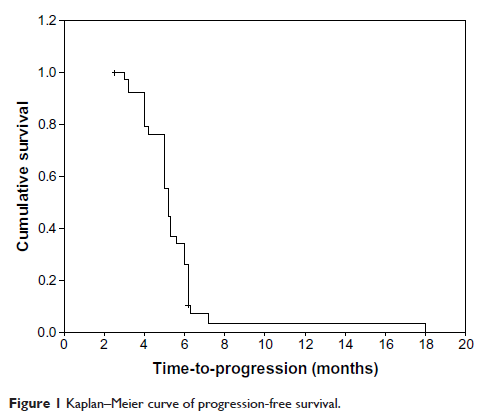
Results: Treatment was well tolerated. Most adverse events were mild. Grade 3 hematological toxicity occurred in 7.7%. There was one complete response (2.6%) and 12 partial responses (30.7%), giving an overall response rate of 33.3% (95% CI [confidence interval], 21.7–50.8). Median time-to-progression was 5.6 months, and median survival was 13.9 months. One- and 2-year survival rates were 60% and 26%, respectively.
Conclusion: S-1 monotherapy is considered a safe and effective treatment option for recurrent and metastatic nasopharyngeal carcinoma patients after failure of platinum-based chemotherapy.
Keywords: S-1, nasopharyngeal carcinoma, chemotherapy, platinum
Patients and methods: Thirty-nine patients with recurrent and metastatic nasopharyngeal carcinoma who failed previous platinum-based chemotherapy received oral S-1 chemotherapy (twice daily from day 1 to 14) every 3 weeks. The dose of S-1 was determined according to the body surface area (BSA): 40 mg twice a day for BSA <1.25 m2; 50 mg twice a day for 1.25 m2 ≤BSA<1.5 m2; and 60 mg twice a day for BSA ≥1.5 m2.
Results: Treatment was well tolerated. Most adverse events were mild. Grade 3 hematological toxicity occurred in 7.7%. There was one complete response (2.6%) and 12 partial responses (30.7%), giving an overall response rate of 33.3% (95% CI [confidence interval], 21.7–50.8). Median time-to-progression was 5.6 months, and median survival was 13.9 months. One- and 2-year survival rates were 60% and 26%, respectively.
Conclusion: S-1 monotherapy is considered a safe and effective treatment option for recurrent and metastatic nasopharyngeal carcinoma patients after failure of platinum-based chemotherapy.
Keywords: S-1, nasopharyngeal carcinoma, chemotherapy, platinum