109814
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

LINC00612/miR-31-5p/Notch1 轴可调节由香烟烟雾提取物诱导的人肺微血管内皮细胞的凋亡、炎症和氧化应激
Authors Luo J, Li L, Hu D, Zhang X
Received 2 April 2020
Accepted for publication 21 July 2020
Published 26 August 2020 Volume 2020:15 Pages 2049—2060
DOI https://doi.org/10.2147/COPD.S255696
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
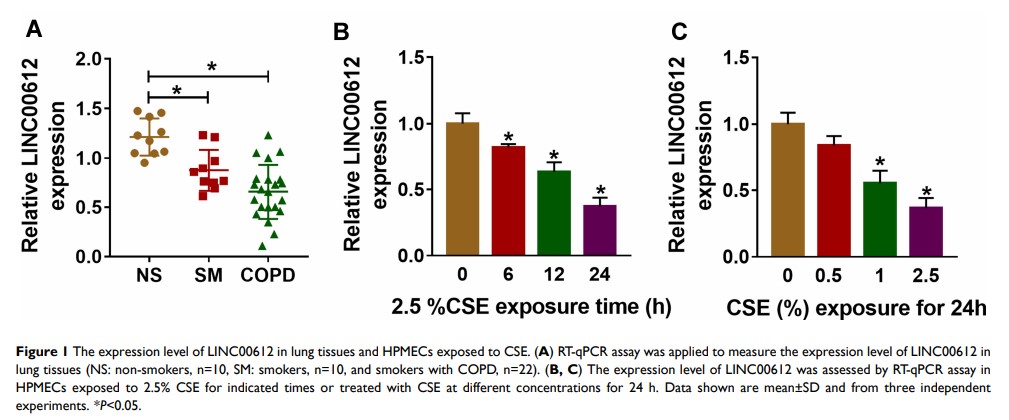
Background: Long non-coding RNAs (lncRNAs) have been reported as key regulators in chronic obstructive pulmonary disease (COPD). However, the precise role of LINC00612 remains unclear.
Methods: The real-time quantitative polymerase chain reaction (RT-qPCR) was used to quantify the expression levels of LINC00612, miR-31-5p, and Notch homolog 1 (Notch1) in lung tissues and cells. Under a cigarette smoke extract (CSE) stimulation condition, the apoptosis was analyzed by flow cytometry assay. Caspase-3 activity was examined with a caspase-3 activity assay kit; besides, inflammation and oxidative stress were assessed by measuring interleukin-6, tumor necrosis factor-α, glutathione/oxidized glutathione, reactive oxygen species, malondialdehyde, and superoxide dismutase activity. The interaction relationship between miR-31-5p and LINC00612 or Notch1 was predicted by bioinformatics databases, while dual-luciferase reporter, RNA immunoprecipitation, and RNA pull-down assays were performed to confirm prediction. Eventually, the related protein expression was estimated with western blot assay.
Results: LINC00612 was downregulated in COPD tissues when compared with controls. Consistently, CSE inhibited LINC00612 expression in HPMECs with a dose/time-dependent method. Gain-of-function experiments indicated that the upregulation of LINC00612 could repress cell apoptosis, inflammation, and oxidative stress in HPMECs induced by CSE. In addition, miR-31-5p was negatively regulated by LINC00612 in HPMECs treated with CSE. The overexpression of miR-31-5p could abolish LINC00612-induced effects on HPMECs exposed to CSE. Importantly, LINC00612 could weaken CSE-induced cell apoptosis, inflammation, and oxidative stress in HPMECs by regulating the miR-31-5p/Notch1 signaling pathway.
Conclusion: Current findings suggest that CSE-mediated cell apoptosis, inflammation, and oxidative stress in HPMECs were abolished by upregulation of LINC00612. Furthermore, the LINC00612/miR-31-5p/Notch1 axis may represent a novel regulator of apoptosis, inflammation, and oxidative stress of HPMECs, which may be a potential therapeutic target for COPD in the future.
Keywords: LINC00612, miR-31-5p, Notch1, COPD, CSE