109814
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

中国健康志愿者空腹和进食条件下盐酸阿米替林片的生物等效性研究
Authors Zhai Y, Wu L, Zheng Y, Wu M, Huang Y, Huang Q, Shentu J, Zhao Q, Liu J
Received 21 April 2020
Accepted for publication 17 July 2020
Published 4 August 2020 Volume 2020:14 Pages 3131—3142
DOI https://doi.org/10.2147/DDDT.S258173
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Purpose: This study compares the pharmacokinetic and safety profiles between a new generic and a branded reference formulation of amitriptyline hydrochloride tablets, and assesses the bioequivalence of the two products in healthy Chinese volunteers to obtain sufficient evidence for the marketing approval of the generic drug.
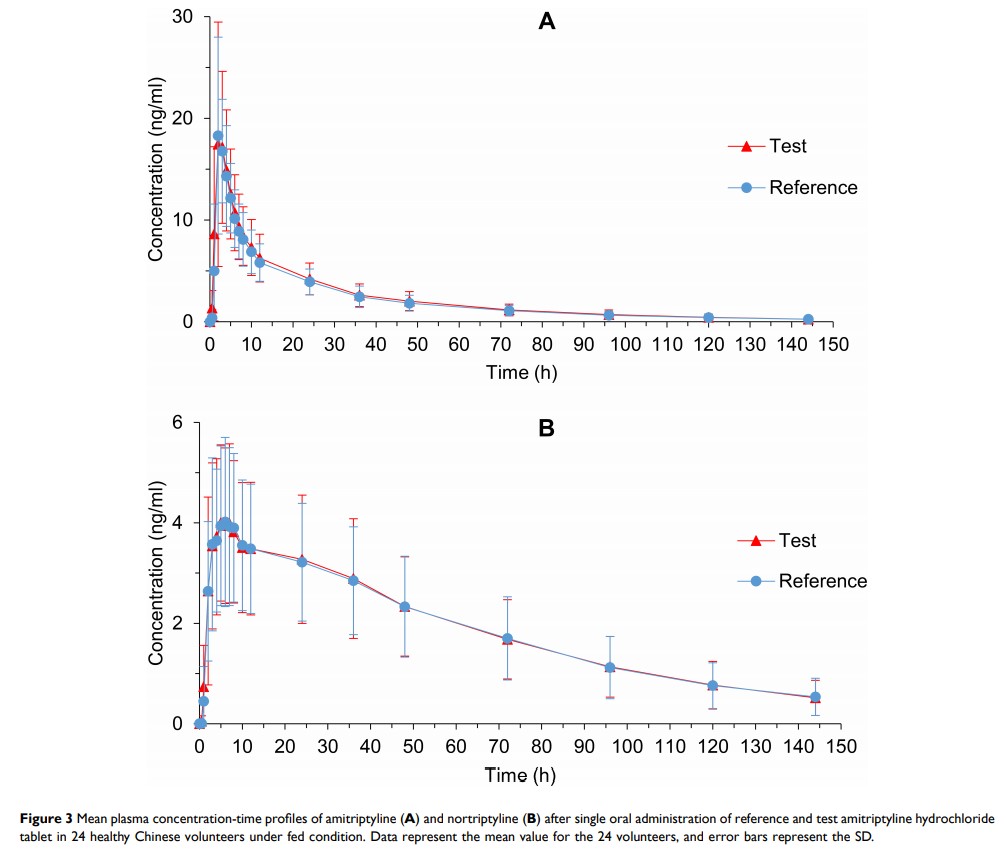
Materials and Methods: A randomized, open-label, two-period crossover study (clinicaltrials.gov, NCT03646526) was conducted under both fasting and fed conditions in healthy Chinese volunteers (24 subjects/condition). Eligible subjects randomly received a single 25 mg dose of either the test or the reference formulation, followed by a 3-week washout period. Blood samples were collected until 144 h following administration. The pharmacokinetic parameters were acquired based on the concentration-time profiles, including the areas under the plasma concentration-time curve (AUC0-t, AUC0-∞), the peak plasma concentration (Cmax), the time to achieve Cmax (Tmax), and the elimination half-life (t1/2). The geometric mean ratios (GMRs) and the corresponding 90% confidence intervals (CIs) of amitriptyline were acquired for bioequivalence analysis, and values of these parameters for nortriptyline were used for comparison of therapeutic outcomes. Safety assessments included laboratory tests, physical examination, vital signs, and incidence of adverse events (AEs).
Results: The values of t1/2 and Tmax for amitriptyline were not significantly different between the test and reference products under both fasting and fed conditions (P > 0.05). The GMRs of Cmax, AUC0–t, and AUC0-∞ between the two products, and corresponding 90% CIs, were all within the range of 80% to 125% under both fasting and fed conditions. The test and reference products were well tolerated and did not elicit serious adverse events.
Conclusion: This study demonstrated that the generic and reference products were well tolerated by the subjects and bioequivalent, according to the rate and extent of the drug absorption.
Keywords: bioequivalence, pharmacokinetics, amitriptyline hydrochloride, nortriptyline