109814
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

OIP5-AS1/miR-137/ZNF217 轴可促进上皮性卵巢癌的恶性行为
Authors Guo L, Chen J, Liu D, Liu L
Received 7 November 2019
Accepted for publication 8 June 2020
Published 3 August 2020 Volume 2020:12 Pages 6707—6717
DOI https://doi.org/10.2147/CMAR.S237726
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Background: Long non-coding RNAs (lncRNAs) have been reported to play crucial regulatory roles in cellular activities and are associated with the carcinogenesis of various diseases. OIP5-AS1, as a novel lncRNA, function in epithelial ovarian cancer (EOC) still remains unclear.
Material and Methods: qRT-PCR and Western blot analyses were performed to measure relevant expression, as needed. A series of functional experiments were performed to determine the role of OIP5-AS1 in EOC cells. Luciferase report, RNA pull down and RIP assays were performed to testify the interaction between relevant RNAs.
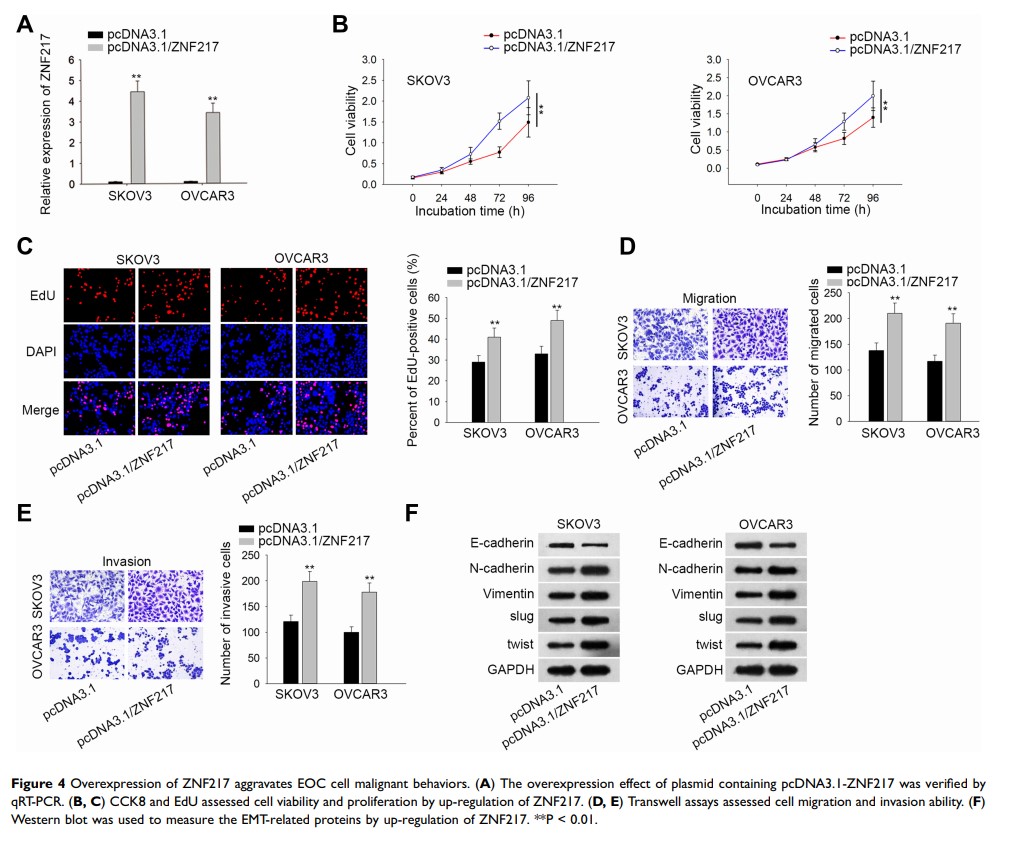
Results: We found that OIP5-AS1 was significantly overexpressed in EOC. Knockdown of OIP5-AS1 inhibited cell proliferation, migration, invasion and epithelial–mesenchymal transition (EMT) process, yet facilitated apoptosis in vitro. OIP5-AS1 functioned as a competing endogenous RNA (ceRNA) to elevate ZNF217 expression through sponging miR-137. Furthermore, miR-137 inhibition and ZNF217 upregulation can reverse the effects of silencing OIP5-AS1 on the cellular activities of ovarian cancer cells. Also, depleted OIP5-AS1 hindered tumor growth and metastasis in vivo.
Conclusion: OIP5-AS1 regulated ovarian cancer progression via modulating miR-137/ZNF217 signaling, suggesting that targeting OIP5-AS1 could be conducive to EOC clinical treatment.
Keywords: OIP5-AS1, miR-137, ZNF217, epithelial ovarian cancer