109814
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

通过摧毁牙龈卟啉单胞菌生物膜相关基因来获得碳量子点特定的抑制生物膜活性
Authors Liang G, Shi H, Qi Y, Li J, Jing A, Liu Q, Feng W, Li G, Gao S
Received 11 March 2020
Accepted for publication 9 June 2020
Published 31 July 2020 Volume 2020:15 Pages 5473—5489
DOI https://doi.org/10.2147/IJN.S253416
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
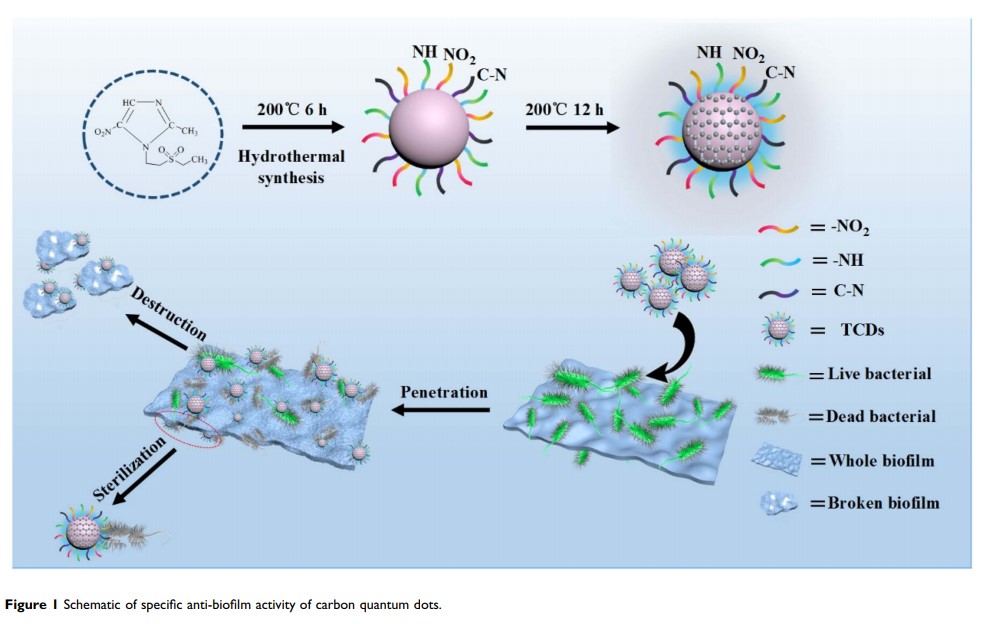
Introduction: Biofilms protect bacteria from antibiotics and this can produce drug-resistant strains, especially the main pathogen of periodontitis, Porphyromonas gingivalis . Carbon quantum dots with various biomedical properties are considered to have great application potential in antibacterial and anti-biofilm treatment.
Methods: Tinidazole carbon quantum dots (TCDs) and metronidazole carbon quantum dots (MCDs) were prepared by a hydrothermal method with the clinical antibacterial drugs tinidazole and metronidazole, respectively. Then, TCDs and MCDs were characterized by transmission electron microscopy, UV–visible spectroscopy, infrared spectroscopy and energy-dispersive spectrometry. The antibacterial effects were also investigated under different conditions.
Results: The TCDs and MCDs had uniform sizes. The results of UV–visible and energy-dispersive spectrometry confirmed their important carbon polymerization structures and the activity of the nitro group, which had an evident inhibitory effect on P. gingivalis , but almost no effect on other bacteria, including Escherichia coli , Staphylococcus aureus and Prevotella nigrescens . Importantly, the TCDs could penetrate the biofilms to further effectively inhibit the growth of P. gingivalis under the biofilms. Furthermore, it was found that the antibacterial effect of TCDs lies in its ability to impair toxicity by inhibiting the major virulence factors and related genes involved in the biofilm formation of P. gingivalis , thus affecting the self-assembly of biofilm-related proteins.
Conclusion: The findings demonstrate a promising new method for improving the efficiency of periodontitis treatment by penetrating the P. gingivalis biofilm with preparations of nano-level antibacterial drugs.
Keywords: P. gingivalis , carbon dots, Tinidazole, biofilms, penetration