109814
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

用于前列腺癌治疗、加载卡巴他赛的牛血清白蛋白纳米粒子的制备和评价
Authors Wan Z, Xie F, Wang L, Zhang G, Zhang H
Received 19 April 2020
Accepted for publication 15 July 2020
Published 29 July 2020 Volume 2020:15 Pages 5333—5344
DOI https://doi.org/10.2147/IJN.S258856
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Purpose: Cabazitaxel (CBZ) is a new taxane-based antitumor drug approved by the FDA for the treatment of prostate cancer, especially for patients with advanced prostate cancer for whom docetaxel is ineffective or causes aggravation. However, Tween 80 injection can cause serious allergic reactions, and CBZ itself has strong toxicity, adverse reactions, and poor tumor selectivity, which greatly limits its clinical applications. Therefore, the CBZ-loaded bovine serum albumin nanoparticles (CBZ-BSA-Gd-NPs) were developed to overcome the allergenic response of Tween 80 and realize the integration of diagnosis and treatment.
Methods: CBZ-BSA-Gd-NPs were prepared by the biomineralization method. The characterization, magnetic resonance imaging (MRI), safety, and antitumor activity of the nanoparticles were evaluated in vitro and in vivo.
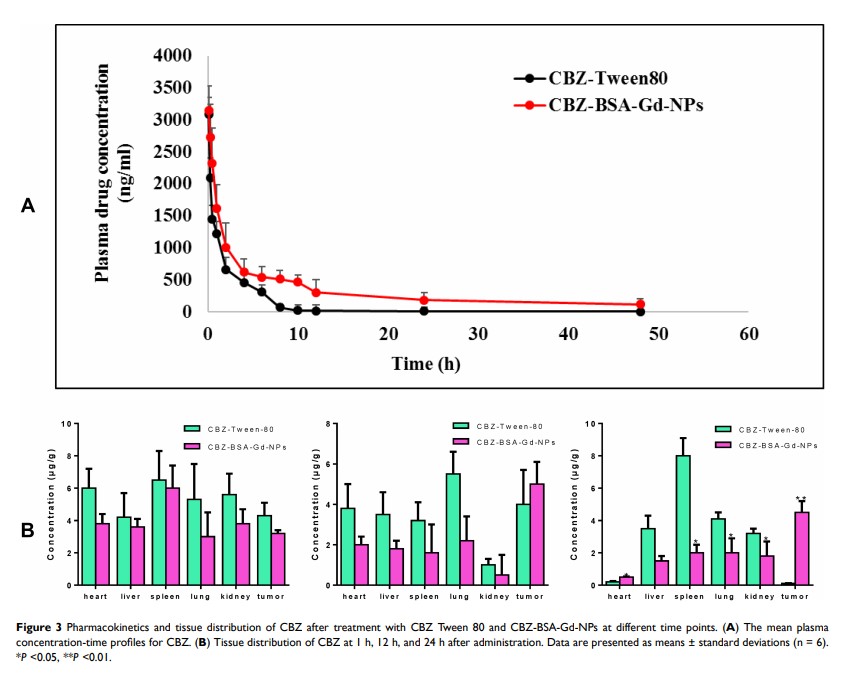
Results: The prepared nanoparticles were uniform in size (166 nm), with good MRI performance and stability over 24 h. Compared with CBZ-Tween 80 injection, CBZ-BSA-Gd-NPs showed much lower hemolysis, similar tumor inhibition, and enhanced cellular uptake in vitro. The pharmacokinetic behavior of CBZ-BSA-Gd-NPs in rats showed that the retention time of the nanoparticles was prolonged, the clearance rate decreased, and the area under the drug-time curve increased. The distribution of CBZ-BSA-Gd-NPs in nude mice was characterized by UPLC-MS/MS and MRI, and the results showed that CBZ-BSA-Gd-NPs could effectively target tumor tissues with reduced distribution in the heart, liver, spleen, lungs, and kidneys compared with CBZ-Tween 80, which indicated that CBZ-BSA-Gd-NPs not only had a passive targeting effect on tumor tissue but also achieved the integration of diagnosis and treatment. In vivo, CBZ-BSA-Gd-NPs showed improved tumor inhibitory effect with a safer profile.
Conclusion: In summary, CBZ-BSA-Gd-NPs can serve as an effective therapeutic drug carrier to deliver CBZ into prostate cancer, and realize the integration of diagnosis and therapy.
Keywords: cabazitaxel, bovine serum albumin, nanoparticles, prostate cancer, magnetic resonance imaging, gadolinium