109814
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

具有较高化疗诱导的恶心和呕吐风险的胃肠道肿瘤患者,在接受中度致吐性化疗时使用奥氮平三联止吐方案的疗效:一项回顾性研究
Authors Wu X, Wu J, Tong G, Cheng B, Chen M, Yu S, He L, Li Z, Wang S
Received 18 March 2020
Accepted for publication 13 July 2020
Published 29 July 2020 Volume 2020:12 Pages 6575—6583
DOI https://doi.org/10.2147/CMAR.S254398
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lu-Zhe Sun
Purpose: Dexamethasone combined with 5-hydroxytryptamine type 3 receptor antagonists (5-HT3 RA) dual regimen is the standard prophylaxis regimen for patients receiving moderately emetogenic chemotherapy (MEC). However, it has been found in real-world practice that chemotherapy-induced nausea and vomiting (CINV) remains poorly controlled among patients with gastrointestinal tumor, especially in those with high-risk factors for vomiting, such as female, young, and non-alcoholic individuals. Hence, we aimed to evaluate the efficacy of an olanzapine-containing triple regimen in this clinical setting.
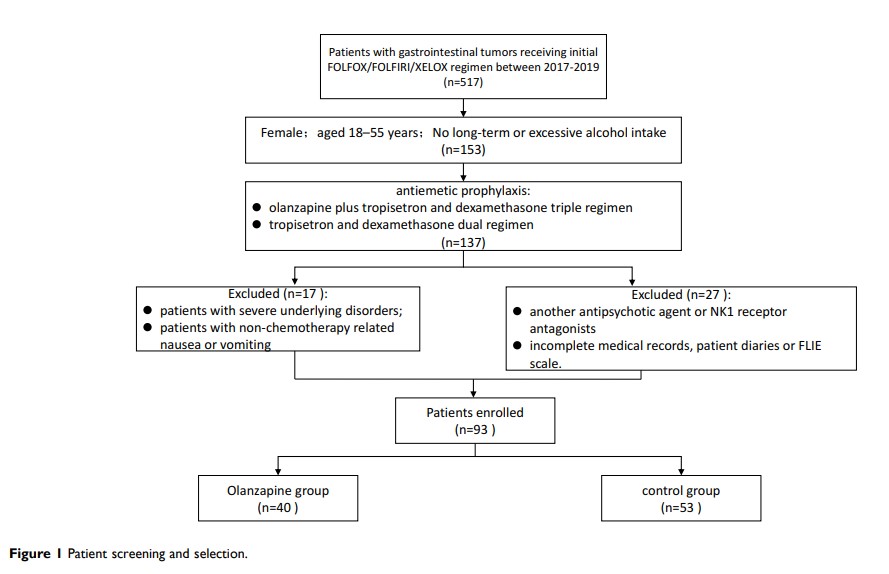
Patients and Methods: We retrospectively reviewed the clinical records of gastrointestinal tumor patients who received mFOLFOX6, XELOX, or FOLFIRI chemotherapy at two institutions. All patients included were female and less than 55 years old, with no history of drinking. The patients were divided into two groups for olanzapine-containing triple therapy (olanzapine, tropisetron, and dexamethasone) and non-olanzapine dual therapy (tropisetron and dexamethasone). The study outcomes were complete response (CR), complete control (CC), nausea control, and quality of life (QoL) by the functional living index-emesis (FLIE) questionnaire.
Results: A total of 93 patients were included in the study (olanzapine: 40; control: 53). The CR rate in the olanzapine group was significantly higher than that in the control group in delayed and overall phase (75.0% vs 54.7%, p=0.044; 70.0% vs 47.2%; p=0.028). The CC rate in the overall phase was also better in the olanzapine group (62.5% vs 39.6%, p=0.029). The control of nausea in the overall phase showed a superior trend in the olanzapine group (p=0.059). The olanzapine group exhibited higher FLIE scores, which demonstrated better QoL. More patients in the olanzapine group exhibited somnolence and dizziness. Conversely, the incidence of insomnia and anorexia in the olanzapine group was lower.
Conclusion: This retrospective study indicates that in gastrointestinal tumor patients with high-risk factors for CINV who were receiving MEC, olanzapine-containing triple antiemetic regimen exhibit better efficacy and QoL as compared to non-olanzapine dual regimen. Further randomized studies are required to confirm these results.
Keywords: CINV, MEC, olanzapine, gastrointestinal tumor, high risk of emesis