109568
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

运用 UPLC-MS/MS 及药代动力学研究同时测定比格犬血浆中的帕瑞昔布及其代谢产物伐地昔布的浓度
Authors Li S, Zhu Y, Zhu C, Li S, Li Z, Qiu X
Received 7 August 2019
Accepted for publication 3 March 2020
Published 13 March 2020 Volume 2020:14 Pages 1117—1125
DOI https://doi.org/10.2147/DDDT.S226349
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
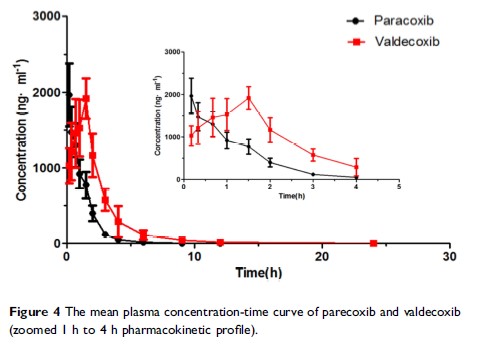
Abstract: A method for the simultaneous determination of parecoxib and its metabolite valdecoxib in beagle plasma by UPLC-MS/MS was developed and validated. After the plasma was extracted by acetonitrile precipitation, the analytes were separated on an Acquity UPLC BEH C18 column (2.1 mm × 50 mm, 1.7 μm) using acetonitrile-formic acid as the mobile phase in gradient mode. The analytes were monitored by multiple reaction monitoring (MRM) in electrospray negative ion mode. The mass transfer pairs were m/z 368.97→ 119.01 for parecoxib, m/z 312.89→ 118.02 for valdecoxib, and m/z 379.98→ 316.02 for celecoxib (internal standard, IS). The correlation coefficients of parecoxib and valdecoxib ranged from 5 to 4000 ng/mL were greater than 0.9998. The recovery of parecoxib and valdecoxib was greater than 82.54%. The inter- and intra-day precision RSD values were 1.36∼ 3.65% and 2.28∼ 5.91%, respectively. The accuracy of RE values were − 1.38%∼ 1.96%. Finally, the matrix effect (ME) and stability were also within acceptable criteria. This method had been successfully applied to the pharmacokinetics of parecoxib and valdecoxib in beagle plasma after injection of parecoxib (1.33 mg/kg, intramuscular injection).
Keywords: parecoxib, valdecoxib, UPLC-MS/MS, pharmacokinetics