109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

CDH1 在乳腺癌治疗中的临床意义和潜在药物靶向: 汇总分析和文献回顾
Authors Huang R, Ding P, Yang F
Received 19 April 2015
Accepted for publication 22 May 2015
Published 18 September 2015 Volume 2015:9 Pages 5277—5285
DOI http://dx.doi.org/10.2147/DDDT.S86929
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 3
Editor who approved publication: Professor Wei Duan
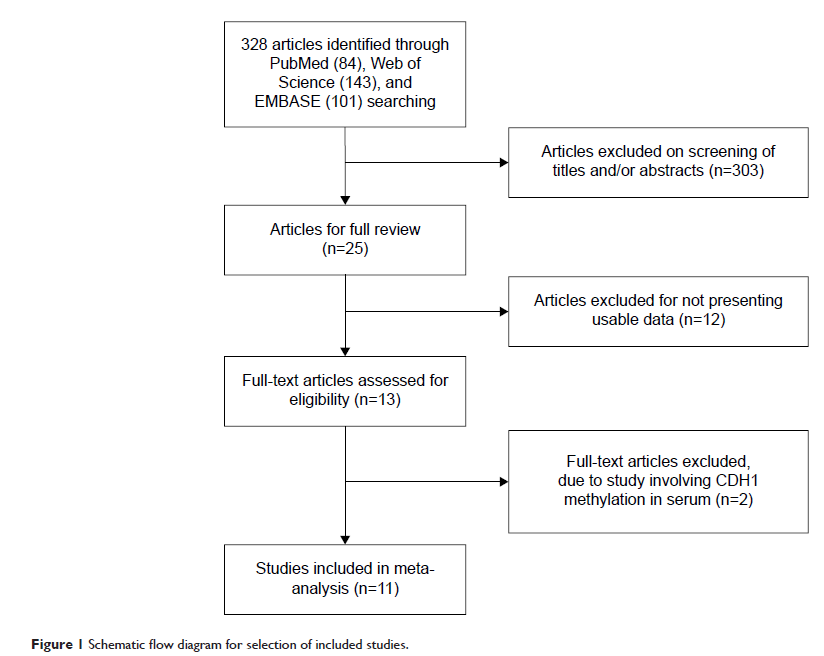
Abstract: CDH1 , as a tumor suppressor gene, contributes sporadic breast cancer (BC) progression. However, the association
between CDH1 hypermethylation and BC, and its clinicopathological significance remains unclear. We conducted a meta-analysis to investigate the relationship between the CDH1 methylation profile and the major clinicopathological features. A detailed literature was searched through the electronic databases PubMed, Web of Science™, and EMBASE™ for related research publications. The data were extracted and assessed by two reviewers independently. Odds ratios (ORs) with corresponding confidence intervals (CIs) were calculated and summarized respectively. The frequency of CDH1 methylation was significantly higher in invasive ductal carcinoma than in normal breast tissues (OR =5.83, 95% CI 3.76–9.03, P <0.00001). CDH1 hypermethylation was significantly higher in estrogen receptor (ER)-negative BC than in ER-positive BC (OR =0.62, 95% CI 0.43–0.87, P =0.007). In addition, we found that the CDH1 was significantly methylated in HER2-negative BC than in HER2-positive BC (OR =0.26, 95% CI 0.15–0.44, P <0.00001). However, CDH1 methylation frequency was not associated with progesterone receptor (PR) status, or with grades, stages, or lymph node metastasis of BC patients. Our results indicate that CDH1 hypermethylation is a potential novel drug target for developing personalized therapy. CDH1 hypermethylation is strongly associated with ER-negative and HER2-negative BC, respectively, suggesting CDH1 methylation status could contribute to the development of novel therapeutic approaches for the treatment of ER-negative or HER2-negative BC with aggressive tumor biology.
Keywords: methylation, estrogen receptor, HER2, triple-negative breast cancer